- Thimerosal is a preservative contained in some vaccines, which has been used and considered safe for decades. During the June 2025 ACIP meeting, a presentation and vote on the use of thimerosal is scheduled to take place.
- During the 2024-2025 respiratory virus season, we found 96% of administered influenza vaccines did not contain thimerosal.
- Low rates of use of thimerosal were seen across age groups and census divisions, with populations under 18 and over 65 having the highest rates of vaccines without thimerosal.
Thimerosal has been used as a preservative in multi-dose vaccine vials for decades to prevent bacterial and fungal contamination (1). Thimerosal is an ethylmercury-based preservative, a different compound than methylmercury (the form of mercury commonly discussed in relation to mercury poisoning, or consuming fish) and is cleared from the body much faster than methylmercury (2). Multiple studies and reviews by leading health authorities, including the Centers for Disease Control and Prevention (CDC), the Food and Drug Administration (FDA), and the World Health Organization (WHO), have consistently found no credible evidence linking thimerosal/ethylmercury to serious health risks, including autism or neurodevelopmental disorders (1, 3–6). Nonetheless, in 1999, the Public Health Service agencies, the American Academy of Pediatrics, and vaccine manufacturers agreed that thimerosal should be reduced or eliminated in vaccines as a precaution (1). Now all influenza vaccines are thimerosal-free with the exception of a few multi-dose vial formulations (4).
Despite a large body of evidence supporting no linkage between thimerosal in vaccines and health risks (5–10), the June 2025 ACIP meeting agenda includes a presentation and corresponding vote on thimerosal in influenza vaccines, which may indicate upcoming changes for influenza vaccine formulations (11). In a climate of increasing distrust regarding public health and medical institutions, it is essential to lead with facts and data.
As public health officials begin to think about influenza vaccine availability in the coming season and Americans try to understand the impact of this meeting, we began asking an important question: how many people have received thimerosal-containing influenza vaccines in past respiratory virus seasons?
Methods
Using a subset of Truveta Data, we identified a cohort of people who received influenza shots between August 2018 and March 2025. We used CPT and CVX codes to identify influenza vaccines that contained thimerosal (the multi-dose vial formulations) or that we could confirm did not contain thimerosal. Vaccines with a composition that could not be determined, such as nonspecific codes for ‘influenza vaccine, not otherwise specified’ were not included. Our analysis only used CPT and CVX codes and thus includes vaccines administered in a clinical/medical setting and recorded in the patient’s EHR and likely does not capture vaccines administered in pharmacies or through public health departments.
We identified the respiratory virus season during which each vaccine was administered (i.e., August to March of the following year). Vaccines administered outside of these months were not included in the analysis.
We calculated the percentage of vaccines that contained thimerosal each respiratory virus season. We also looked at trends by census division and age groups in the 2024-2025 season.
You can view the entire study—including data definitions, code, and more—directly in Truveta Studio.
Results
We included 36,813,590 administered influenza vaccines where it could be determined if the vaccine contained thimerosal.
Overall
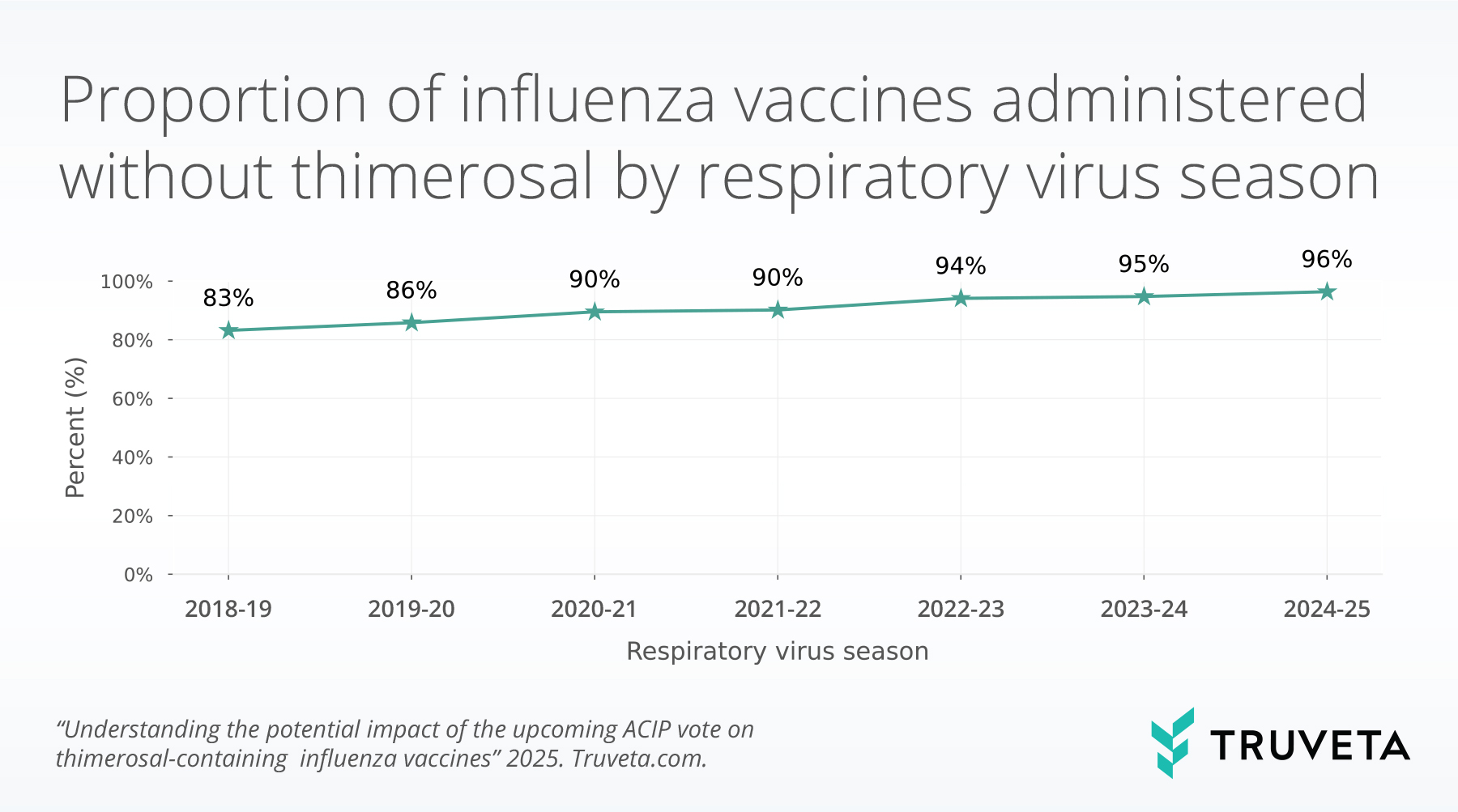
The rate of administered vaccines containing thimerosal has steadily decreased since the 2018-2019 respiratory virus season. The rate of influenza vaccines without thimerosal was 83% in 2018-2019 and this increased to 96% in the 2024-2025 respiratory virus season.
Regional trends
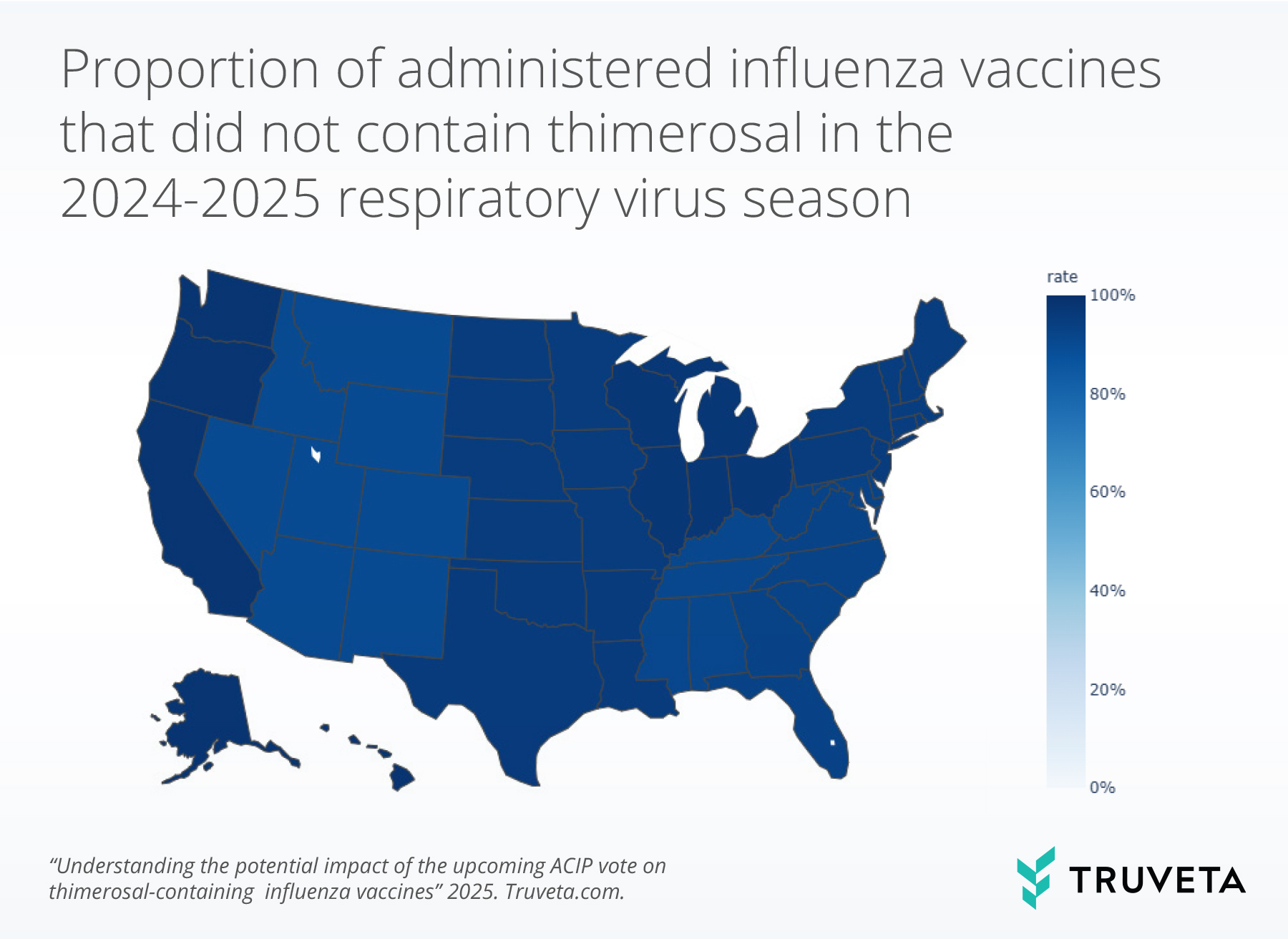
The census division trends mimicked the overall trends. In 2024-2025 respiratory virus season, over 90% of vaccines did not contain thimerosal in all census divisions.
Age groups
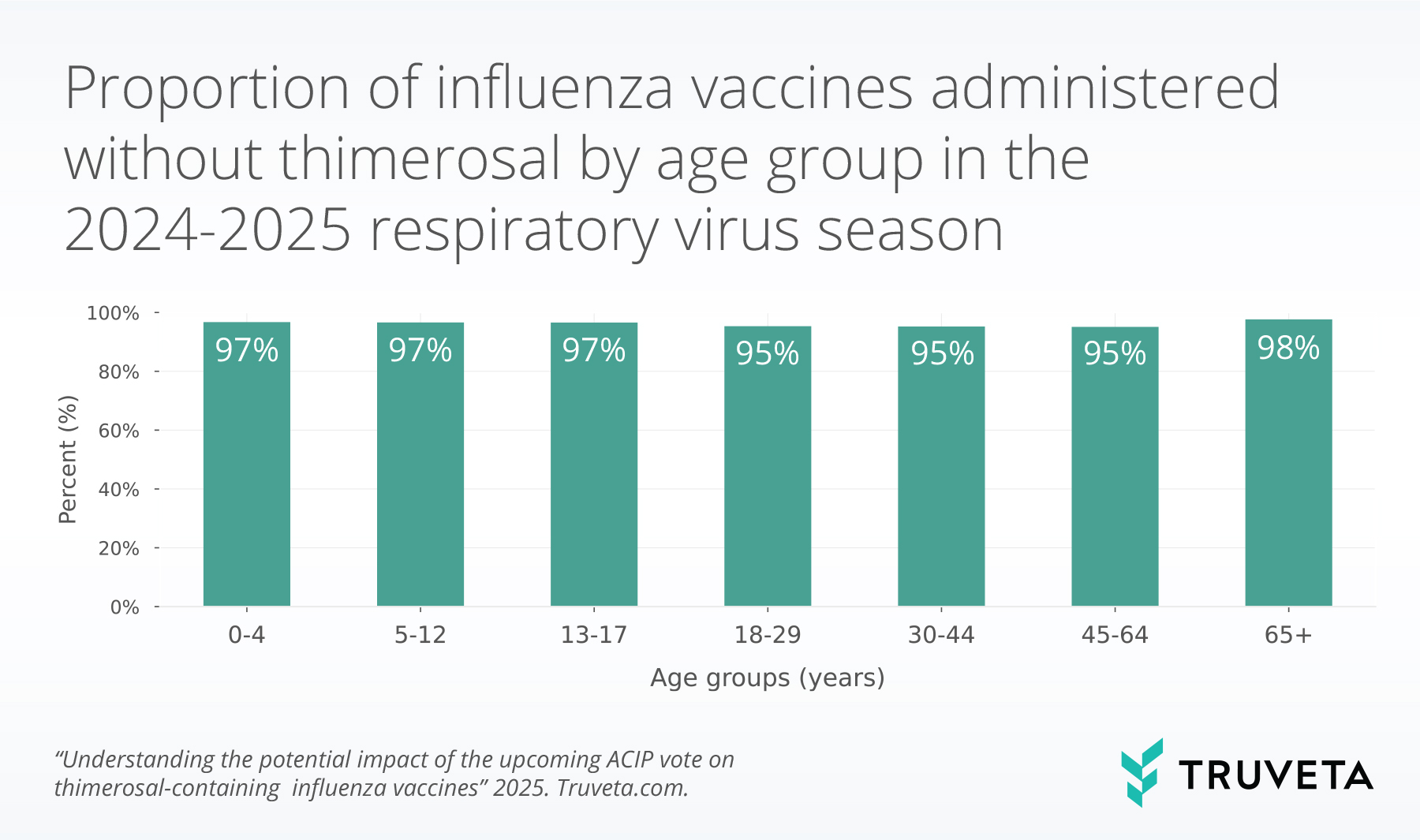
In the 2024-2025 respiratory virus season, the rate of influenza vaccines that did not contain thimerosal was over 95% for all age groups.
The highest rates of thimerosal-free influenza vaccines (97%) were in age groups under 18 years old, with the exception of people over the age of 65 (98%).
Discussion
The upcoming presentation and vote on thimerosal-containing influenza vaccines at the June 2025 ACIP meeting prompted this retrospective analysis into the frequency of use of multi-dose vaccine formulations containing this preservative in recent respiratory virus seasons. Although a large body of scientific literature has found no credible link between thimerosal and health risks (5–10)—including no association with autism or neurodevelopmental disorders—amid a broader climate of skepticism toward medical and public health institutions, distrust of vaccines continues to proliferate.
The findings of this report show that thimerosal-containing influenza vaccines have become increasingly rare in recent years. During the 2024–2025 respiratory virus season, 4% of administered influenza vaccines contained thimerosal, continuing a clear downward trend since the 2018–2019 season. This pattern was consistent across all US census divisions and age groups, suggesting that the market has already shifted substantially toward thimerosal-free formulations, consistent with the 1999 policy change to remove thimerosal where possible.
These findings suggest that any upcoming recommendation to remove or further restrict thimerosal-containing influenza vaccines would affect a small portion of influenza vaccines administered in a medical/clinic setting in the United States, though the corresponding ripple effects regarding public trust in vaccines may limit vaccine uptake and confidence.
One main limitation in this analysis is we used of CPT and CVX codes to define thimerosal containing and preservative-free vaccines, which likely excludes vaccines administered outside of a healthcare setting (such as those dispensed in pharmacies). We expect that our results are representative of the influenza vaccines administered in the healthcare setting, but our results cannot be generalized to those administered in pharmacies or public health vaccine drives.
Our analysis provides valuable context for interpreting the potential policy change under consideration by the ACIP by highlighting the rarity of thimerosal-containing influenza vaccines in use in the US and underscores the power of real-world data in contextualizing public health policy.
These are preliminary research findings and not peer reviewed. Data are constantly changing and updating. These findings are consistent with data accessed on June 25, 2025.
You can view the entire study—including data definitions, code, and more—directly in Truveta Studio.
Citations
- Centers for Disease Control and Prevention, Thimerosal and Vaccines (2024). https://www.cdc.gov/vaccine-safety/about/thimerosal.html.
- United States Environmental Protection Agency, Health Effects of Exposures to Mercury (2024). https://www.epa.gov/mercury/health-effects-exposures-mercury#methyl.
- World Health Organization, Thimerosal (2025). https://www.who.int/teams/health-product-policy-and-standards/standards-and-specifications/norms-and-standards/vaccine-standardization/thiomersal.
- U.S. Food & Drug Administration, Thimerosal and Vaccines (2025). https://www.fda.gov/vaccines-blood-biologics/safety-availability-biologics/thimerosal-and-vaccines.
- C. S. Price, W. W. Thompson, B. Goodson, E. S. Weintraub, L. A. Croen, V. L. Hinrichsen, M. Marcy, A. Robertson, E. Eriksen, E. Lewis, P. Bernal, D. Shay, R. L. Davis, F. DeStefano, Prenatal and Infant Exposure to Thimerosal From Vaccines and Immunoglobulins and Risk of Autism. Pediatrics 126, 656–664 (2010).
- P. Stehr-Green, P. Tull, M. Stellfeld, P.-B. Mortenson, D. Simpson, Autism and thimerosal-containing vaccines. American Journal of Preventive Medicine 25, 101–106 (2003).
- W. W. Thompson, C. Price, B. Goodson, D. K. Shay, P. Benson, V. L. Hinrichsen, E. Lewis, E. Eriksen, P. Ray, S. M. Marcy, J. Dunn, L. A. Jackson, T. A. Lieu, S. Black, G. Stewart, E. S. Weintraub, R. L. Davis, F. DeStefano, Early Thimerosal Exposure and Neuropsychological Outcomes at 7 to 10 Years. N Engl J Med 357, 1281–1292 (2007).
- A. E. Tozzi, P. Bisiacchi, V. Tarantino, B. De Mei, L. D’Elia, F. Chiarotti, S. Salmaso, Neuropsychological Performance 10 Years After Immunization in Infancy With Thimerosal-Containing Vaccines. Pediatrics 123, 475–482 (2009).
- T. Verstraeten, R. L. Davis, F. DeStefano, T. A. Lieu, P. H. Rhodes, S. B. Black, H. Shinefield, R. T. Chen, Vaccine Safety Datalink Team, Safety of thimerosal-containing vaccines: a two-phased study of computerized health maintenance organization databases. Pediatrics 112, 1039–1048 (2003).
- L. K. Ball, R. Ball, R. D. Pratt, An Assessment of Thimerosal Use in Childhood Vaccines. Pediatrics 107, 1147–1154 (2001).
- Centers for Disease Control and Prevention\, “MEETING OF THE ADVISORY COMMITTEE ON IMMUNIZATION PRACTICES (ACIP)” (2025); https://www.cdc.gov/acip/downloads/agendas/Final-posted-2025-06-24-508.pdf.

