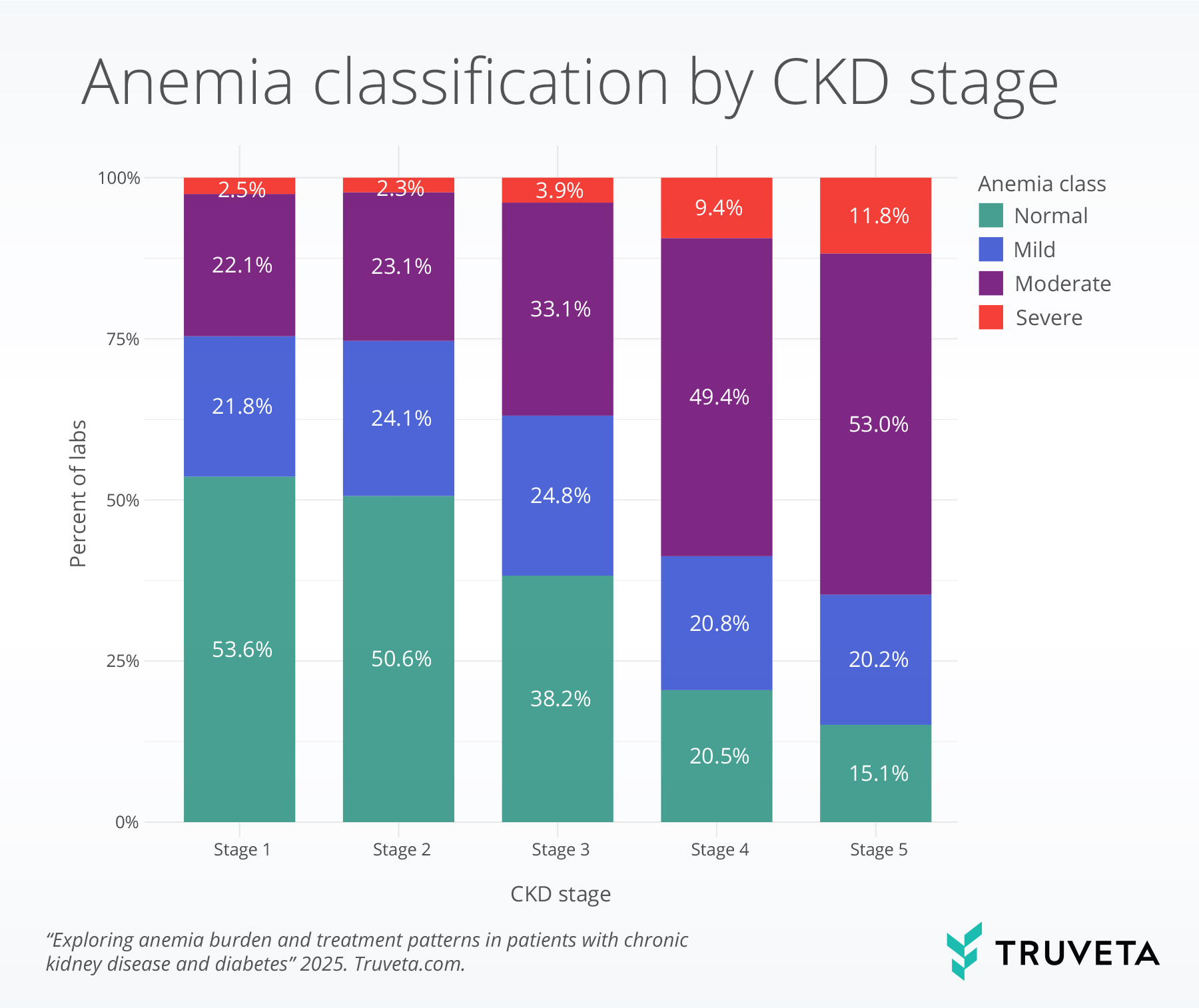
- In patients with CKD and diabetes, anemia prevalence and severity increased from stage 1 to stage 5, with overall anemia rising from 46.4% to 85.0% and severe anemia from 2.5% to 11.8%
- Erythropoiesis-stimulating agents (ESAs) and IV iron use rose as hemoglobin levels dropped, though with distinct stage-specific patterns and evidence of flexible application of guideline thresholds.
Anemia is a shortage of healthy, oxygen-carrying red blood cells, which leads to fatigue, weakness, and shortness of breath. It is a common complication of chronic kidney disease (CKD), particularly among those with diabetes (1, 2). is the leading cause of CKD and together they create a compounded risk for both the development and severity of anemia (3, 4). In CKD, the kidneys lose their ability to produce erythropoietin, a hormone essential for red blood cell production (1).
Anemia in patients with both CKD and diabetes significantly increases the risk of cardiovascular disease, accelerates the progression of kidney failure, and is associated with higher rates of hospitalization and mortality (7–9). Effective management is therefore essential, and current clinical practice recommendations are outlined in the Kidney Disease: Improving Global Outcomes (KDIGO) guidelines (10).
According to the KDIGO guidelines, anemia in patients with CKD is diagnosed when hemoglobin levels fall below 13 g/dL in men or 12 g/dL in women (7, 10). If iron deficiency is present, oral or is the recommended first-line treatment. If anemia persists despite adequate iron stores and hemoglobin remains below 10 g/dL, erythropoiesis-stimulating agents (ESAs) may be initiated to support red blood cell production (10, 11).
In this report, we examined hemoglobin lab results from patients with CKD and type 1 or type 2 to describe the prevalence and severity of anemia across CKD stage, dialysis status, and patient characteristics. We further evaluated ESA and IV iron use following low hemoglobin values to assess how treatment patterns vary in relation to clinical guidelines and disease stage. The combination of the linked EHR and medical claims data can provide a detailed and more complete view of patient care.
Methods
We used a subset of Truveta Data to identify adults aged 18 years and older with evidence of CKD and type 1 or type 2 diabetes (henceforth referred to as diabetes) between January 2019 and August 2025. CKD and diabetes were defined by the presence of at least one diagnostic code for CKD or diabetes, identified from EHR or medical/pharmacy claims data, recorded prior to the hemoglobin lab. Patients were required to have at least one hemoglobin lab, with continuous medical and pharmacy enrollment for at least six months prior to the test and extending through 90 days afterward. To ensure continuous care, patients were also required to have at least one healthcare encounter in the year preceding the hemoglobin lab.
To ensure that anemia could be attributed to CKD, we excluded hemoglobin lab results if patients had a diagnosis of anemia due to acute blood loss or underwent surgery within 90 days before or 7 days after the test. In addition, patients with a diagnosis of cancer at any time, or who had a live birth recorded within 9 months of their hemoglobin lab were excluded.
Anemia severity classification
Anemia severity was categorized based on hemoglobin levels using sex-specific thresholds (12):
- Severe: <8 g/dL
- Moderate: 8–10.9 g/dL
- Mild: 11–12.9 g/dL (men), 11–11.9 g/dL (women)
- Normal: 13–17.9 g/dL (men), 12–15.9 g/dL (women)
If patients had multiple hemoglobin tests on the same day, the median value was used. Any same-day labs that deviated by more than 1 g/dL from the median value were excluded. Hemoglobin labs with values above the normal reference range were excluded, as the analysis focused on comparing normal versus varying degrees of anemia.
Comorbidities and other patient characteristics
We characterized patients using demographic and clinical data from both EHR and claims sources. CKD severity was assessed by stage, when available, using diagnosis codes. Dialysis status was defined as any dialysis procedure code documented prior to the hemoglobin test. Demographic variables included sex and race, both obtained from EHR data. Comorbid conditions–including chronic obstructive pulmonary disease (COPD), cardiovascular diseases (CVD), hyperlipidemia, and hypertension—were identified using diagnosis codes from EHR and claims data.
Anemia treatment outcomes
We identified the use of ESAs and IV Iron, either through prescription or administration, within 90 days following a low hemoglobin lab. To avoid counting the same treatment multiple times when patients had several labs in a short period, we only used the hemoglobin value closest to the treatment date.
Statistical analysis
We reported the distribution of anemia severity across patients with CKD and diabetes, stratified by CKD stage, dialysis status, sex, age, and race. We used an ordinal logistic regression model to examine the odds of having more severe anemia based on a range of factors. These factors included whether a patient had one of several common chronic conditions (COPD, CVD, hyperlipidemia, and hypertension), as well as age, race, sex, kidney disease stage, and dialysis history. We only present results for factors that were significantly associated with anemia severity in the model.
Among patients with anemia, we estimated the proportions of those who received an ESA or IV iron treatment within 90 days of the low hemoglobin lab. We used 10 g/dL as a reference threshold based on clinical guidelines for initiation of ESA. Although IV iron is typically prescribed based on iron indices (e.g., ferritin or transferrin saturation) rather than hemoglobin values, for consistency with the ESA analysis, 10 g/dL was also used as a reference threshold.
Results
We included 883,067 hemoglobin laboratory results from 124,328 patients with CKD and diabetes, with a median of 3 tests per patient. The mean age at testing was 62 years (SD 14), and 48.7% of lab tests were conducted in male patients. Most hemoglobin labs were from White patients (50.9%), 33.3% were from Black or African American patients (henceforth referred to as Black), 5.9% were from Asian patients, and 18.4% were from patients of Hispanic ethnicity.
At the time of hemoglobin testing, the distribution of CKD stage was as follows:
- Stage 1: 3.3%
- Stage 2: 14.8%
- Stage 3: 49.2%
- Stage 4: 9.5%
- Stage 5: 23.1%
Prior dialysis was recorded in 21.5% of labs. Based on hemoglobin values, one third of labs (33.5%) were in the normal range. About 1 in 5 (23.2%) showed mild anemia, while 37.4% showed moderate anemia, and 5.9% showed severe anemia. The median hemoglobin value across all labs was 11.4 g/dL.
Anemia classification by CKD stage
The proportion of normal hemoglobin labs declined with advancing CKD stage, falling from 53.6% of labs at stage 1 to just 15.1% at stage 5. In parallel, severe anemia became more frequent, rising from about 2.5% at stage 1 to 11.8% at stage 5. Moderate anemia accounted for half of labs in stages 4 and 5.
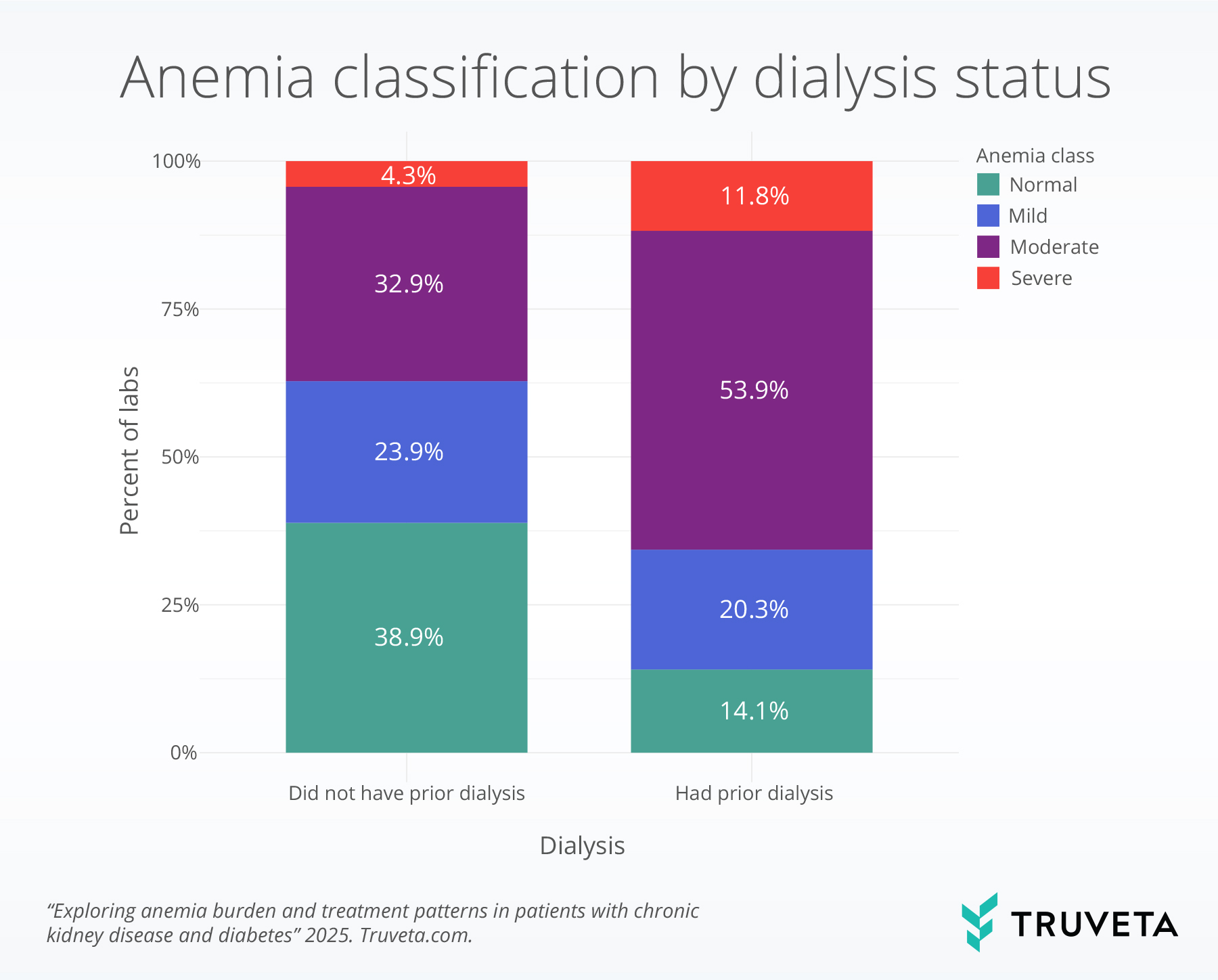
Anemia classification by dialysis status
The severity of anemia differed markedly by dialysis status, with more severe anemia occurring in patients with dialysis. Normal hemoglobin values were observed in 38.9% of patients who had no record of dialysis at the time of testing compared to 14.1% of patients who had a record of dialysis at the time of testing. In contrast, severe anemia occurred in 4.3% of labs from patients not on dialysis compared to 11.8% of labs from those on dialysis. Moderate anemia was also more dominant in patients on dialysis than those not on dialysis, comprising over half of labs (53.9% vs. 32.9%).
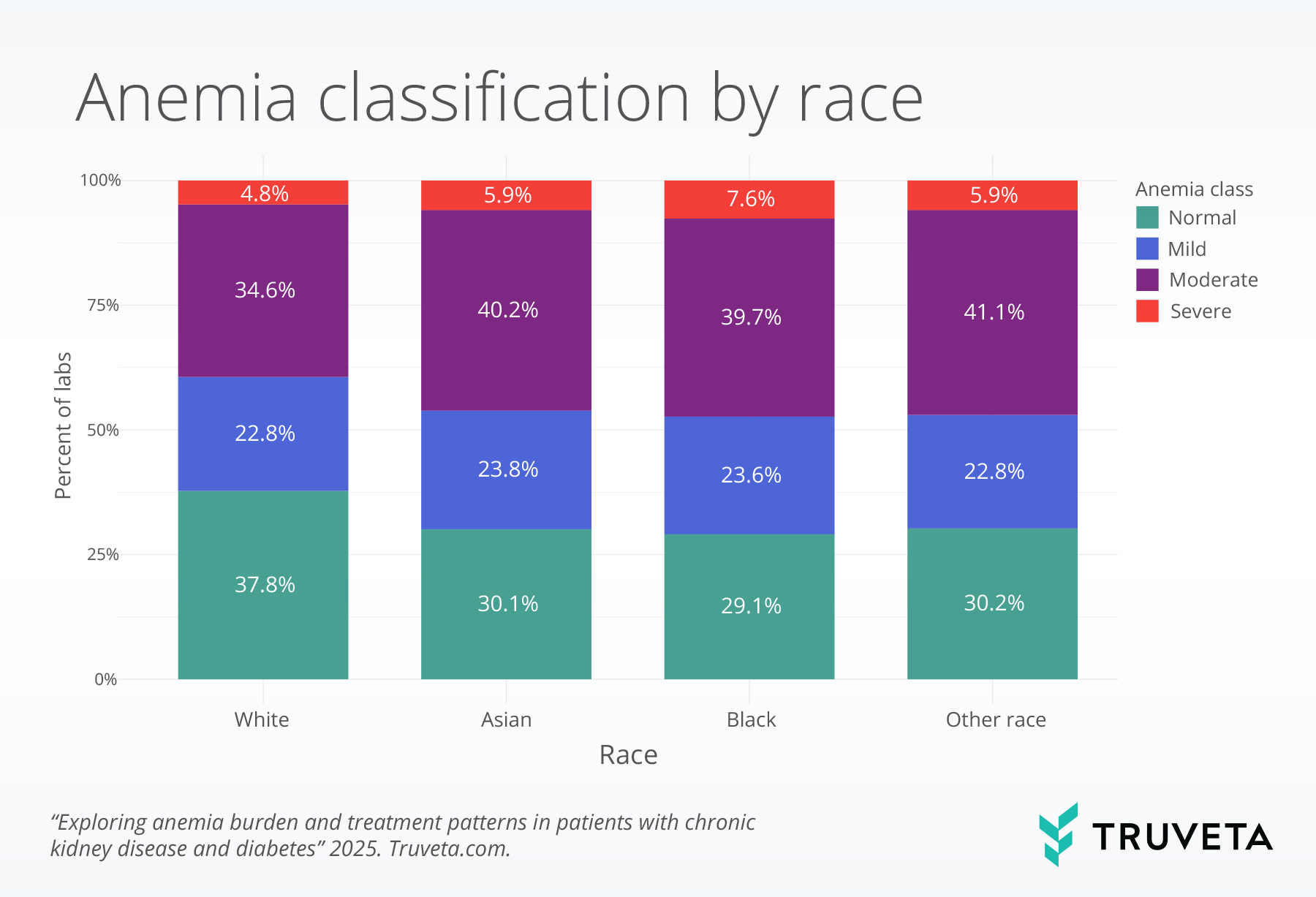
Anemia classification by patient characteristics
Patterns of anemia severity differed modestly between labs from female and male patients. Labs from female patients were more likely to indicate moderate anemia, while labs from male patients were more likely to indicate mild anemia. Patterns of anemia also differed between White and Black patients. Normal hemoglobin values were present in 37.8% of labs from White patients and 29.1% from Black patients. In contrast, severe anemia was more common among Black patients than White patients (7.6 vs. 4.8%), and moderate anemia was slightly more common among Black patients (39.7% vs. 34.6%).
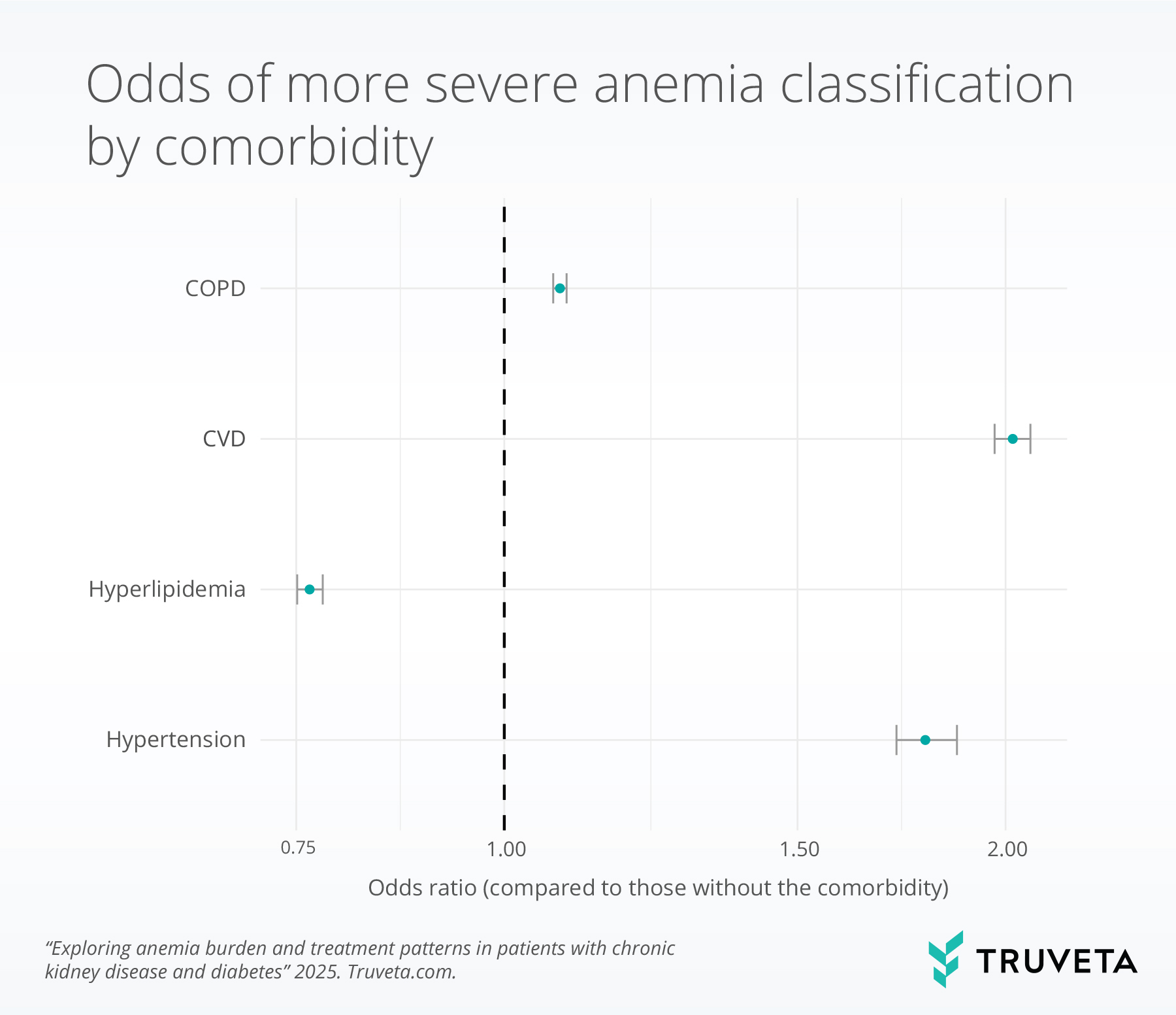
We used an ordinal logistic regression model to compare the odds of being in a more severe anemia category. In this context, an odds ratio (OR) > 1 indicates higher odds of worse anemia compared with the reference group, while an OR < 1 indicates lower odds. When the confidence interval (grey bars in the image below) crosses 1, the association is not statistically significant.
Patients with COPD (OR = 1.08, p<.001), CVD (OR=2.02, p <.001), and hypertension (OR = 1.79, p<.0001) had significantly higher odds of being in a more severe anemia category compared with those without these conditions. In contrast, patients with hyperlipidemia had lower odds of more severe anemia (OR = 0.76, p<.001).
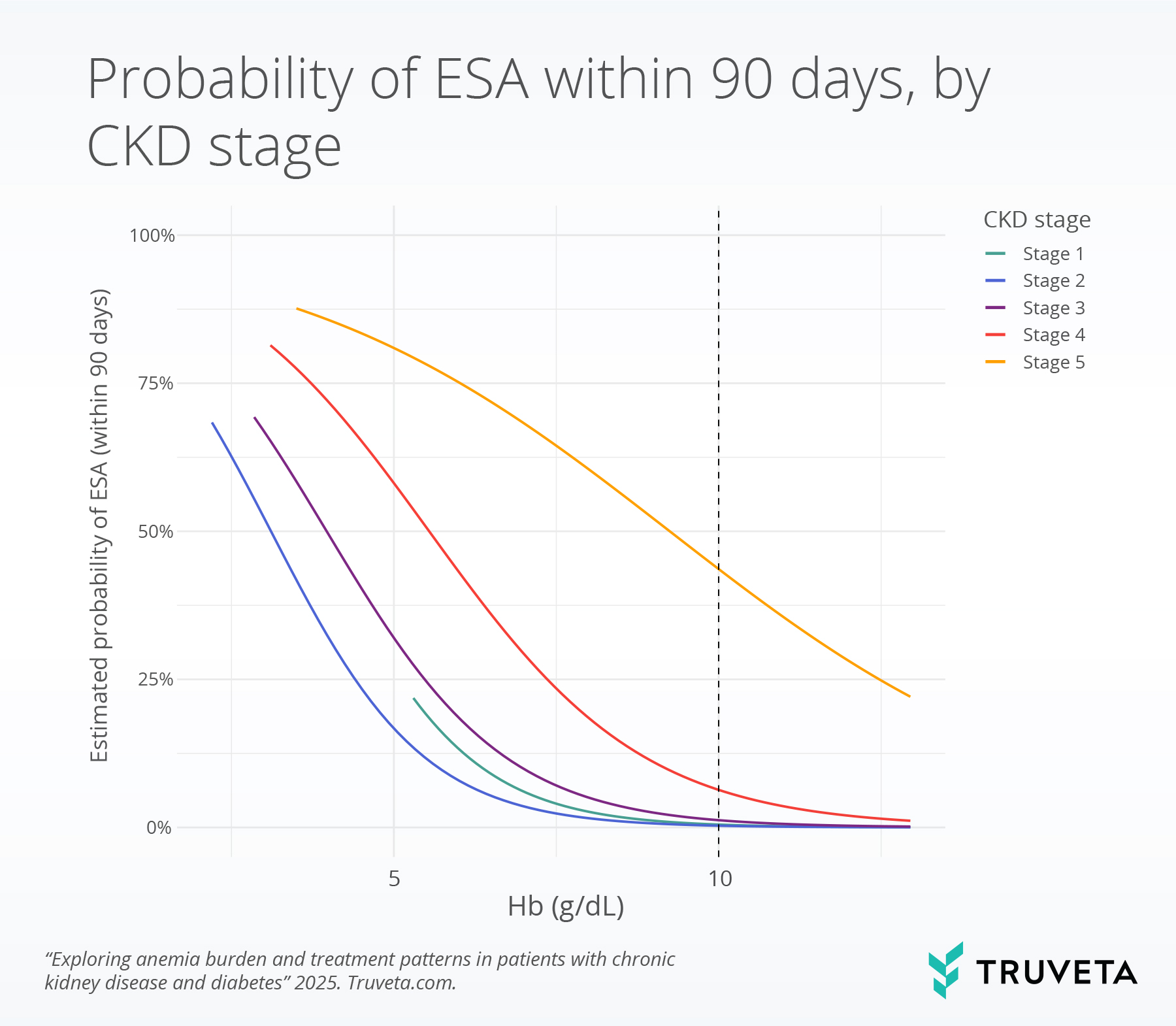
Probability of ESA treatment by CKD stage
The probability of ESA treatment within 90 days rose steeply as hemoglobin levels declined, with 10 g/dL serving as the clinical guideline threshold for initiating treatment. At this threshold, probabilities of ESA treatment were very low in early CKD (0.5% in stage 1, 0.3% in stage 2, and 1.2% in stage 3) but increased to 6.3% in stage 4 and 43.6% in stage 5.
When hemoglobin levels fell below 10 g/dL, patients were more likely to start ESA treatment.
- Stage 3 CKD: ESA use rose gradually—from 1.2% at 10 g/dL, to 2.5% at 9 g/dL, and 5.0% at 8 g/dL.
- Stage 4 CKD: The increase was sharper—from 6.3% at 10 g/dL, to 11.0% at 9 g/dL, and 18.5% at 8 g/dL.
- Stage 5 CKD: ESA treatment was already common at 10 g/dL, and use continued to rise as hemoglobin dropped lower.
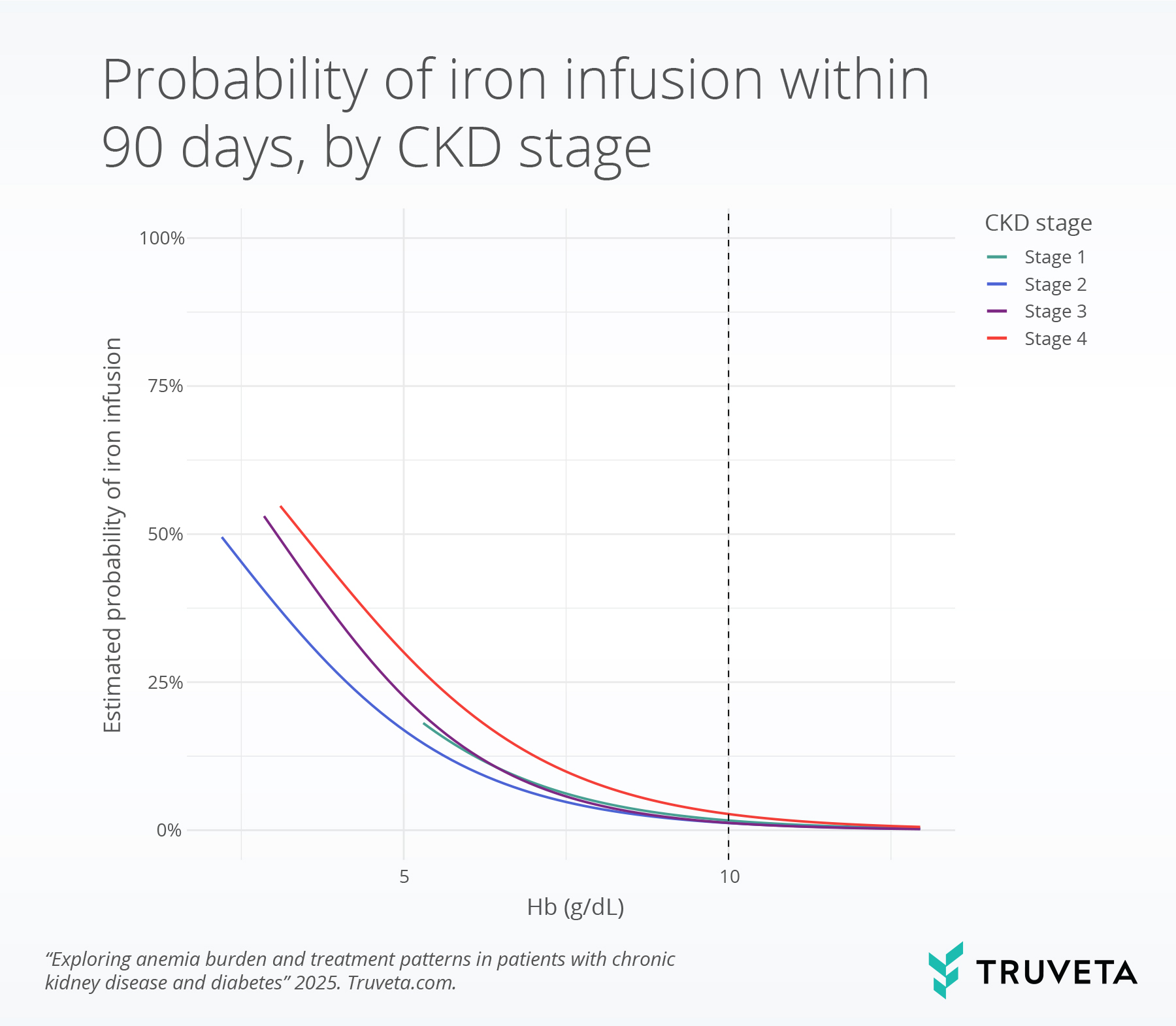
Probability of IV iron infusion by CKD stage
The probability of IV iron treatment within 90 days increased sharply as hemoglobin levels declined. Stage 5 CKD was removed from this analysis because treatment decisions at this stage become substantially more nuanced and complex (10, 13). At a hemoglobin level of 10 g/dL, the chance of receiving IV iron was quite low across all CKD stages: 1.6% in stage 1, 1.2% in stage 2, 1.2% in stage 3, and 2.7% in stage 4.
- Stage 3 CKD: IV iron use rose steadily as hemoglobin dropped—from 1.2% at 10 g/dL, to 7.7% at 7 g/dL, and 22.6% at 5 g/dL.
- Stage 4 CKD: The rise was sharper—from 2.7% at 10 g/dL, to 12.6% at 7 g/dL, and 30.0% at 5 g/dL.
Discussion
In this large cohort of patients with CKD and diabetes, anemia was common across all CKD stages, and its prevalence and severity increased as kidney function declined. By stage 5 CKD, more than two-thirds of hemoglobin labs reflected moderate-to-severe anemia, and only 15.1% of labs were within the normal range. These findings are consistent with population estimates showing rising anemia prevalence with advancing CKD stage (5, 14, 15).
Meaningful differences were also observed across prior dialysis status, sex, race, and comorbidities, evaluated at the time of each lab. Labs from patients on dialysis or with COPD, CVD, or hypertension were more likely to show anemia, particularly in the moderate-to-severe range. Severe anemia was more common in labs among Black patients compared with White patients. These disparities align with prior work describing disproportionate CKD burden and anemia severity among Black patients, shaped by clinical and socioeconomic factors (15, 16). Moderate anemia was more common in labs from women, while mild anemia was more common in labs from men, which is broadly consistent with literature demonstrating higher rates of anemia in women compared to men (17).
Treatments were closely linked to the observed hemoglobin level and CKD stage. For ESAs, although hemoglobin of 10 g/dL represents a common threshold, use in practice increased progressively as hemoglobin declined, with more aggressive treatment observed in advanced CKD. The probability of receiving treatment within 90 days rose sharply at lower hemoglobin values, particularly in later stages, suggesting that clinicians weigh both labs and CKD severity when initiating ESAs. This stage-specific gradient in treatment use highlights a nuanced application of guideline recommendations in clinical practice (10, 18).
IV iron treatment is typically guided by iron indices (such as ferritin or transferrin saturation) rather than hemoglobin itself, but we used hemoglobin as a reference point for comparability. IV iron treatments were uncommon in early CKD stages but became much more likely as the CKD stage got more advanced and anemia became more severe. We did not include patients with stage 5 CKD in this analysis, as treatment decisions regarding iron at this stage are highly complex and individualized, often requiring separate consideration beyond the scope of this report (10, 19). Broadly, these results demonstrate that iron treatment decisions at this stage may be influenced by more complex clinical considerations beyond hemoglobin thresholds alone.
This study has several notable strengths. We analyzed a large, contemporary cohort that included more than 880,000 laboratory results from more than 124,000 patients, providing robust statistical power to characterize anemia patterns across CKD stages. By leveraging both EHR and claims data, we were able to examine not only the prevalence of anemia but also its severity distribution and subsequent treatment decisions. Interventions such as dialysis and IV iron are more likely to occur in specialized centers outside of typical health system EHR capture. Our use of linked EHR and claims data therefore provides a more complete view of CKD anemia management than either data source alone.
However, these results must be interpreted with caution. The 10 g/dL guideline is not an absolute threshold, and real-world practice likely reflects additional considerations not captured here. For example, ESA initiation may depend on the trajectory of hemoglobin decline, the number of consecutive labs below threshold, and the time interval between those labs. We did not capture other management strategies such as oral iron supplementation (prescribed or over the counter), dietary interventions, or blood transfusions, which may affect ESA use. In addition, our analytic cohort consisted only of patients with at least one hemoglobin laboratory result. Because clinicians are more likely to order hemoglobin testing when anemia is suspected or relevant to management, this design likely enriched the cohort for patients at higher risk of anemia. As a result, the prevalence we observed may appear higher than in population-based CKD studies. Lastly, our analytic cohort was somewhat younger than the general CKD and diabetes population, which may limit generalizability. These limitations highlight that our estimates of treatment initiation reflect only a portion of the broader treatment landscape.
Anemia was highly prevalent across CKD stages, with severity increasing as kidney function declined. Both ESA and IV iron use rose as hemoglobin decreased, though with distinct stage-specific patterns that highlight how guideline recommendations are adapted in practice. By leveraging lab-level data linked with claims, this report provides a comprehensive view of anemia management in CKD, while also underscoring the need for further research into treatment decision-making.
These are preliminary research findings and not peer reviewed. Data are constantly changing and updating. These findings are consistent with data accessed on September 29, 2025.
Citations
- J. L. Babitt, H. Y. Lin, Mechanisms of Anemia in CKD. J Am Soc Nephrol 23, 1631–1634 (2012).
- C. Loutradis, A. Skodra, P. Georgianos, P. Tolika, D. Alexandrou, A. Avdelidou, P. A. Sarafidis, Diabetes mellitus increases the prevalence of anemia in patients with chronic kidney disease: A nested case-control study. World J Nephrol 5, 358–366 (2016).
- P. D. Evans, M. W. Taal, Epidemiology and causes of chronic kidney disease. Medicine 43, 450–453 (2015).
- S. Al-Khoury, B. Afzali, N. Shah, A. Covic, S. Thomas, D. J. Goldsmith, Anaemia in diabetic patients with chronic kidney disease—prevalence and predictors. Diabetologia 49, 1183–1189 (2006).
- U. Mehdi, R. D. Toto, Anemia, diabetes, and chronic kidney disease. Diabetes care 32, 1320–1326 (2009).
- D. K. Singh, P. Winocour, K. Farrington, Erythropoietic stress and anemia in diabetes mellitus. Nature Reviews Endocrinology 5, 204–210 (2009).
- M. E. Stauffer, T. Fan, Prevalence of Anemia in Chronic Kidney Disease in the United States. PLoS ONE 9, e84943 (2014).
- K. Iseki, K. Kohagura, Anemia as a risk factor for chronic kidney disease. Kidney International 72, S4–S9 (2007).
- P. T. Vlagopoulos, H. Tighiouart, D. E. Weiner, J. Griffith, D. Pettitt, D. N. Salem, A. S. Levey, M. J. Sarnak, Anemia as a risk factor for cardiovascular disease and all-cause mortality in diabetes: the impact of chronic kidney disease. Journal of the American Society of Nephrology 16, 3403–3410 (2005).
- KDIGO 2025 CLINICAL PRACTICE GUIDELINE FOR ANEMIA IN CHRONIC KIDNEY DISEASE (CKD) (2024). https://kdigo.org/wp-content/uploads/2024/11/KDIGO-2025-Anemia-in-CKD-Guideline_Public-Review-Draft_Nov42024.pdf.
- B. Biju, A. D’cruz, S. Jiby, M. Devassy, R. Jacob, Strategies for Managing Anemia in Chronic Kidney Disease. Journal of Drug Delivery & Therapeutics 14, 92 (2024).
- Haemoglobin concentrations for the diagnosis of anaemia and assessment of severity (2011). https://www.who.int/publications/i/item/WHO-NMH-NHD-MNM-11.1.
- M. M. Wong, C. Tu, Y. Li, R. L. Perlman, R. Pecoits-Filho, A. A. Lopes, I. Narita, H. Reichel, F. K. Port, N. Sukul, Anemia and iron deficiency among chronic kidney disease Stages 3–5ND patients in the Chronic Kidney Disease Outcomes and Practice Patterns Study: often unmeasured, variably treated. Clinical kidney journal 13, 613–624 (2020).
- M. E. Stauffer, T. Fan, Prevalence of Anemia in Chronic Kidney Disease in the United States. PLoS ONE 9, e84943 (2014).
- C. P. Kovesdy, J. R. Davis, I. Duling, D. J. Little, Prevalence of anaemia in adults with chronic kidney disease in a representative sample of the United States population: analysis of the 1999–2018 National Health and Nutrition Examination Survey. Clin Kidney J 16, 303–311 (2023).
- S. L. Saraf, J. Y. Hsu, A. C. Ricardo, R. Mehta, J. Chen, T. K. Chen, M. J. Fischer, L. Hamm, J. Sondheimer, M. R. Weir, X. Zhang, M. Wolf, J. P. Lash, Anemia and Incident End-Stage Kidney Disease. Kidney360 1, 623–630 (2020).
- W. S. Shiferaw, T. Y. Akalu, Y. A. Aynalem, Risk factors for anemia in patients with chronic renal failure: a systematic review and meta-analysis. Ethiopian journal of health sciences 30 (2020).
- R. Raichoudhury, B. S. Spinowitz, Treatment of anemia in difficult-to-manage patients with chronic kidney disease. Kidney International Supplements 11, 26–34 (2021).
- I. C. Macdougall, A. J. Bircher, K.-U. Eckardt, G. T. Obrador, C. A. Pollock, P. Stenvinkel, D. W. Swinkels, C. Wanner, G. Weiss, G. M. Chertow, J. W. Adamson, T. Akizawa, S. D. Anker, M. Auerbach, P. Bárány, A. Besarab, S. Bhandari, I. Cabantchik, A. J. Collins, D. W. Coyne, Á. L. M. de Francisco, S. Fishbane, C. A. J. M. Gaillard, T. Ganz, D. J. Goldsmith, C. Hershko, E. A. Jankowska, K. L. Johansen, K. Kalantar-Zadeh, P. A. Kalra, B. L. Kasiske, F. Locatelli, J. Małyszko, G. Mayer, L. P. McMahon, A. Mikhail, E. Nemeth, A. B. Pai, P. S. Parfrey, R. Pecoits-Filho, S. D. Roger, G. Rostoker, J. Rottembourg, A. K. Singh, I. Slotki, B. S. Spinowitz, D.-C. Tarng, F. Tentori, J. E. Toblli, Y. Tsukamoto, N. D. Vaziri, W. C. Winkelmayer, D. C. Wheeler, E. Zakharova, Iron management in chronic kidney disease: conclusions from a “Kidney Disease: Improving Global Outcomes” (KDIGO) Controversies Conference. Kidney International 89, 28–39 (2016).