Non–small cell lung cancer (NSCLC) accounts for about 85% of lung cancer cases and remains the leading cause of cancer death worldwide. While major advances in targeted therapies and immunotherapies have transformed care, gaps persist in biomarker testing, treatment sequencing, and patient access.
New findings based on Truveta Data highlight these challenges. In a recent analysis of nearly 10,000 patients with metastatic NSCLC, fewer than half received biomarker testing within 90 days of diagnosis, despite guideline recommendations. Testing rates improved between 2018 and 2021 but have since plateaued—leaving many patients without timely access to therapies that could improve outcomes.
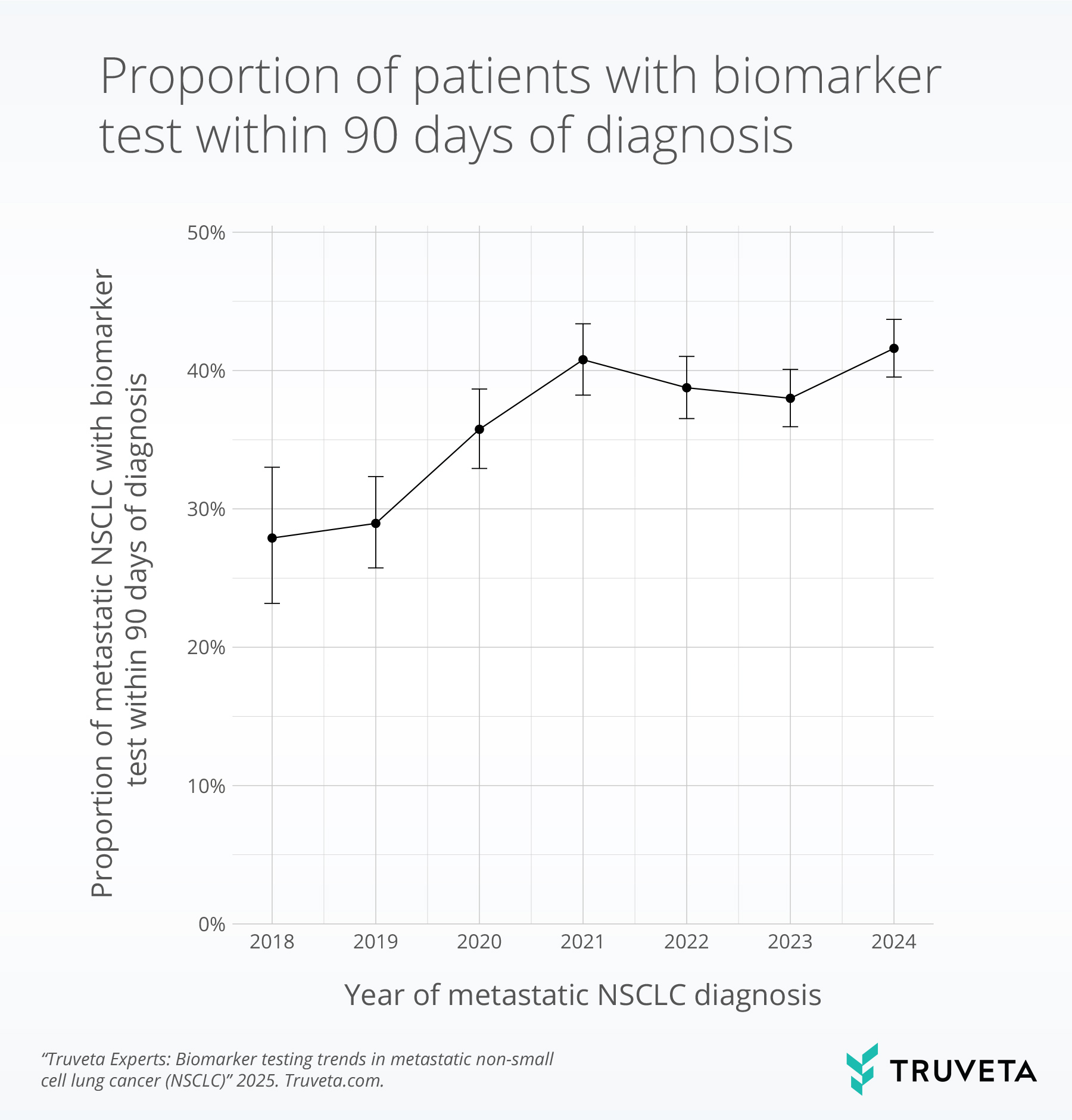
Because Truveta Data is updated daily, including lab values, notes, and outcomes, researchers can see these practice shifts as they happen, not years later.
Understanding NSCLC research needs with real-world data
NSCLC treatment is increasingly biomarker-driven. Patients may receive targeted therapies for EGFR, ALK, ROS1, or KRAS mutations, or immunotherapies guided by PD-L1 status. Others still receive chemotherapy or combinations of regimens. Researchers need real-world evidence to track not only what therapies are used, but also why choices are made, how long treatments are continued, and what outcomes follow.
Truveta Data tracks the full patient journey
For NSCLC, Truveta Data provides:
- Biomarker testing results: EGFR, ALK, ROS1, KRAS, PD-L1, including test timing
- Pathology and staging: Histology subtypes and stage at diagnosis
- Imaging reports: CT, PET, MRI results to follow tumor progression
- Treatment data: Uptake and sequencing of targeted therapies, immunotherapies, and chemotherapy
- Labs: Relevant labs for safety monitoring and treatment decisions
- Clinical notes: Reasons for therapy discontinuation, symptoms, patient reported outcomes, grade and sites of metastatic disease
- Closed claims linkage: Including economics, enrollment history, pharmacy, and medical benefits from all settings of care
- Demographics and SDOH: Age, sex, race, and geography for equity analyses
Clinical notes: Unlocking the “why” in NSCLC care
Structured data alone cannot explain why patients discontinue therapy, switch regimens, or miss biomarker testing. With the Truveta Language Model (TLM), researchers can extract insights from provider notes at scale—such as reasons for stopping immunotherapy, details from imaging reports, or patient-reported side effects.
This helps answer not just what happened in care, but why.
The most representative, complete, and timely EHR data
Truveta provides daily updated EHR data for more than 120 million patients across the US, linked with claims, SDOH, and mortality data. This breadth and depth ensures consistent data quality and full traceability to the clinical source.
For NSCLC, this means studying the entire journey—from diagnostic testing and initial staging, through biomarker-driven treatment, to disease progression and outcomes. Researchers can evaluate adoption of targeted and immunotherapies, compare persistence across regimens, and assess disparities in care.
Looking ahead
NSCLC care is rapidly evolving with the growth of immunotherapies, targeted therapies, and novel approaches like liquid biopsy. As practice guidelines shift, life sciences teams need timely real-world evidence to understand biomarker adoption, treatment sequencing, and outcomes.
Truveta Data enables researchers to answer these questions at scale and in real time—supporting clinical development, HEOR, post-market studies, and health equity initiatives.
Explore more
Contact us for a custom feasibility to see how Truveta can support your NSCLC research strategy.
