Truveta Data
Regulatory grade safety and effectiveness data replacing trials and registries
Clinical trials and registries suffer from lengthy timelines, substantial costs, and limited generalizability
7B+
clinical notes
45M+
unique devices
5+
years
of longitudinal patient history
100M+
imaging studies
120M+
patients and growing
100+
payers
99.6%
coverage of US counties
5+
years
of longitudinal patient history
200M+
closed claims patients and growing
Accurately linked across
50M+
patients and growing
Updated daily, expert de-identified, and immediately available
Explore complete, patient-level data to accelerate therapy adoption
Complete EHR data for 120M patients, including:














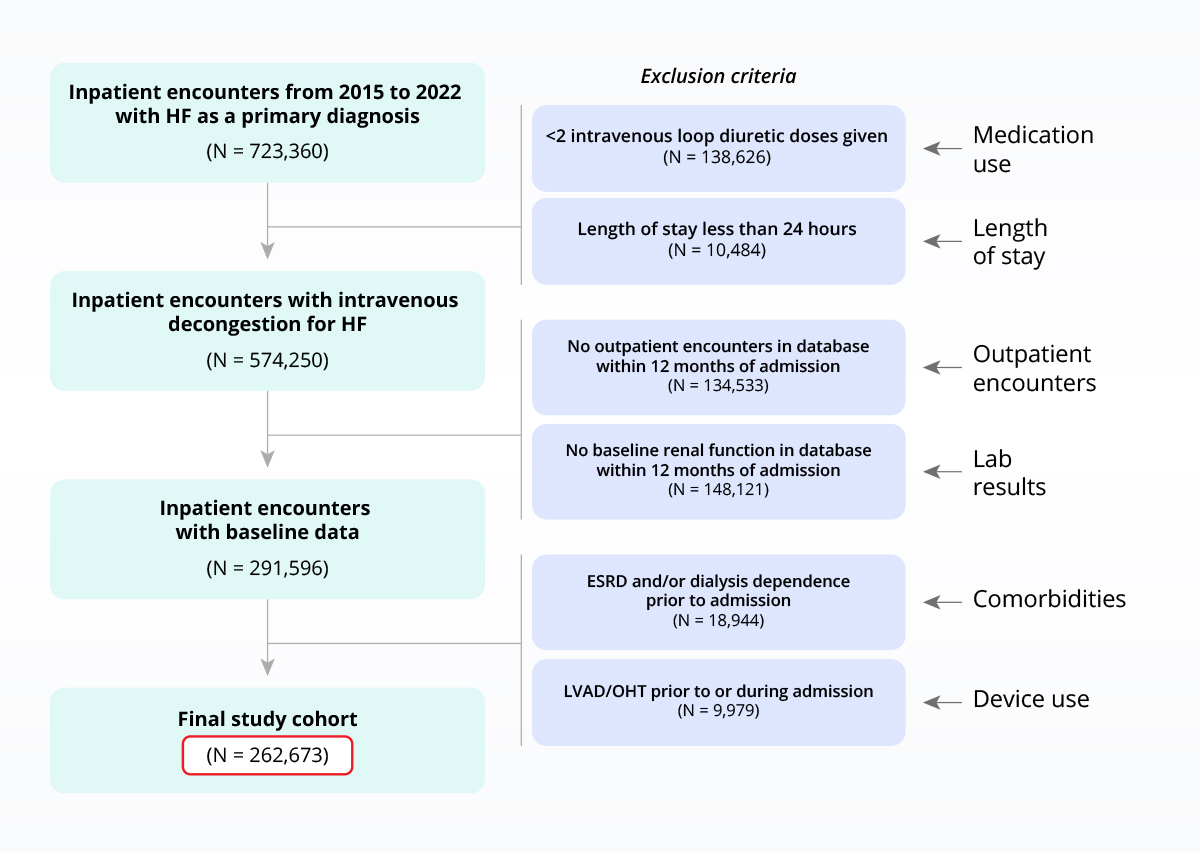
The clinical depth and breadth of Truveta Data enables the use of highly specific inclusion/exclusion criteria.
Access notes and images integrated with EHR data
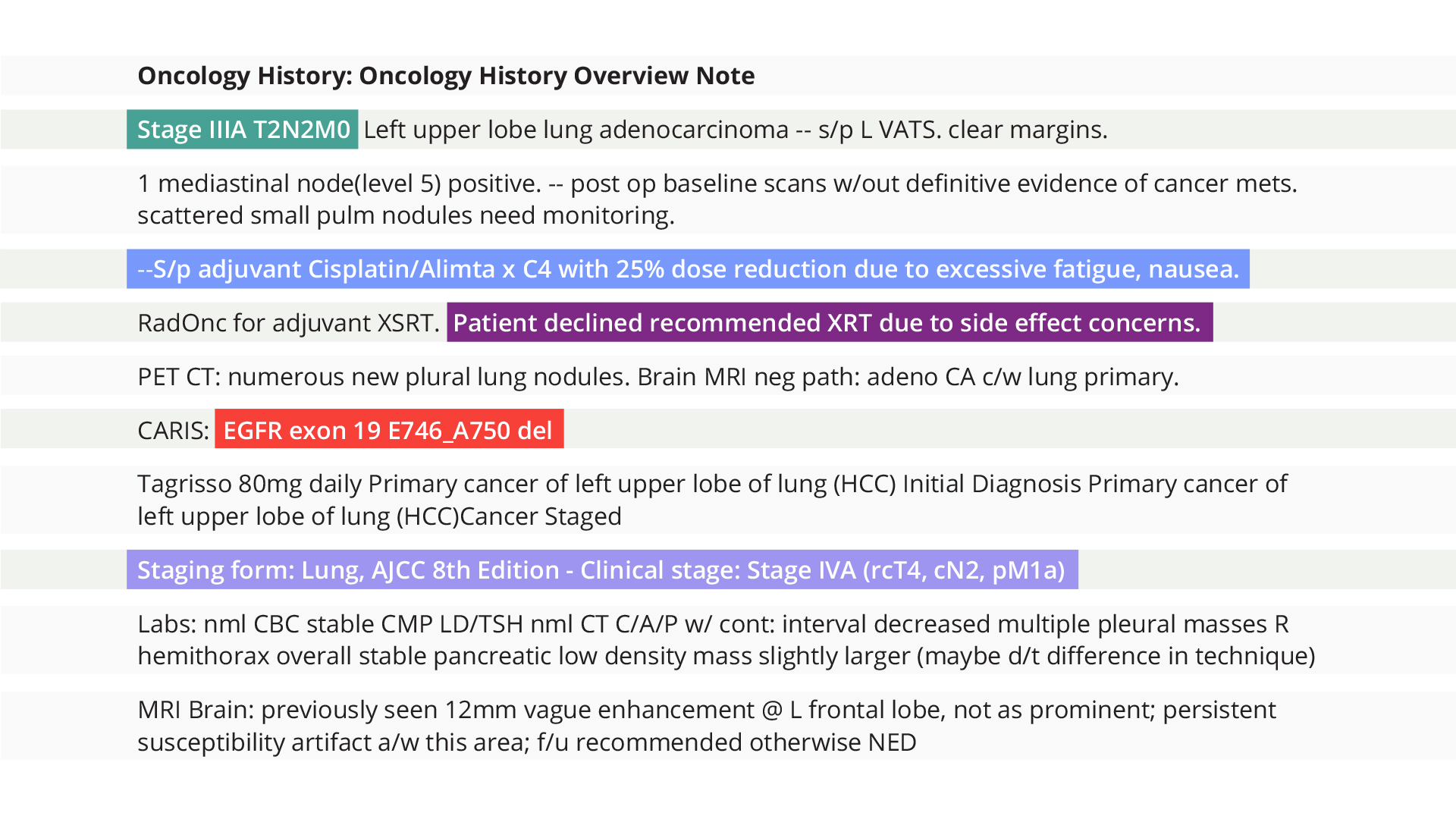
Unlock meaningful data from clinical notes
Understand complete clinical context and pursue novel research with more than 7 billion notes.
Truveta receives all clinical notes generated during a patient’s care. This includes progress notes, nursing evaluations, procedure/operative reports, referral notes, discharge summaries, and more.
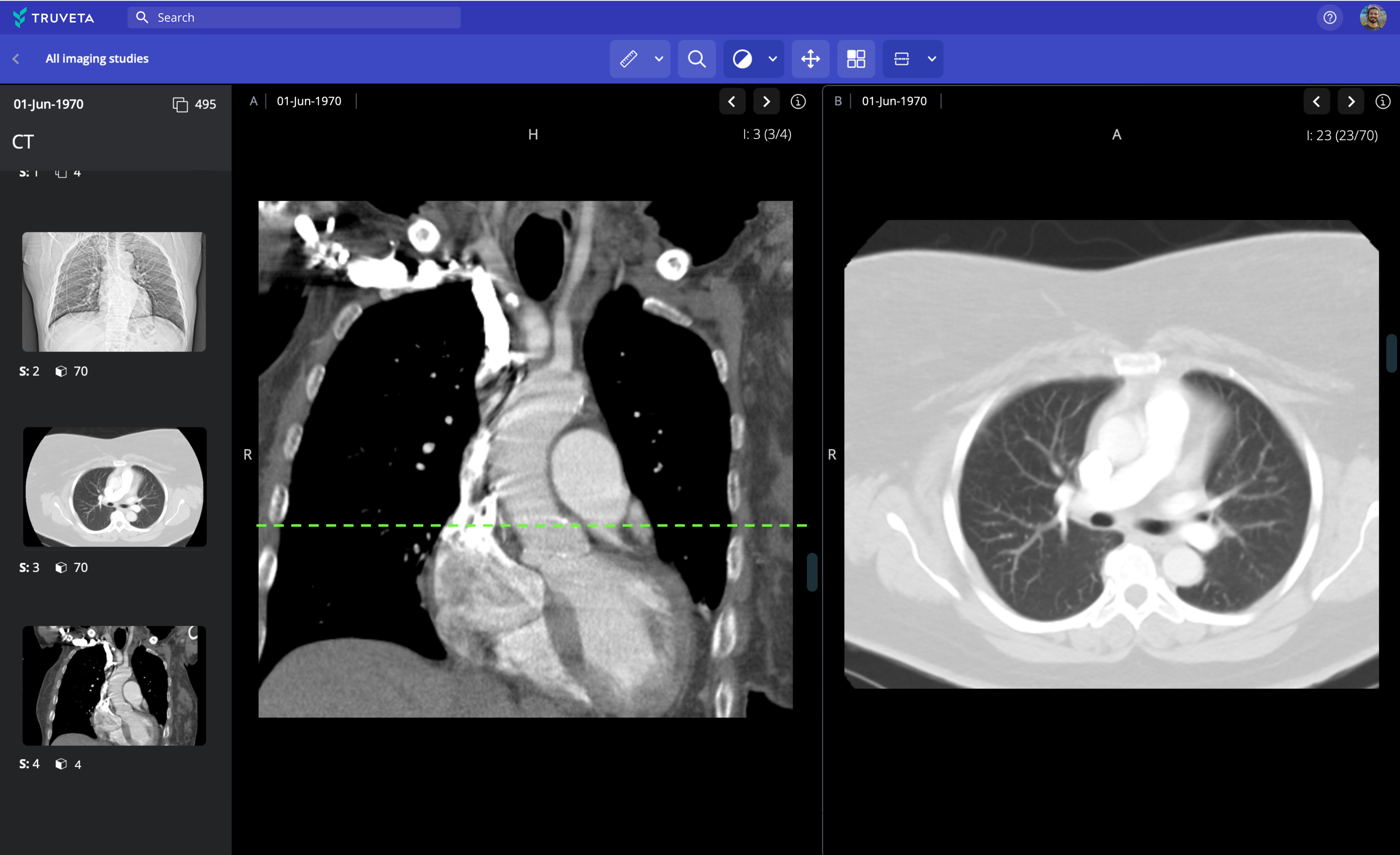
Learn from millions of medical images integrated with complete EHR data
Truveta Data includes medical images across all modalities, including MRI, CT, X-ray, ultrasound, mammogram, PET, and nuclear medicine, searchable by modality and protocol.
Learn more millions of medical images alongside complete EHR data
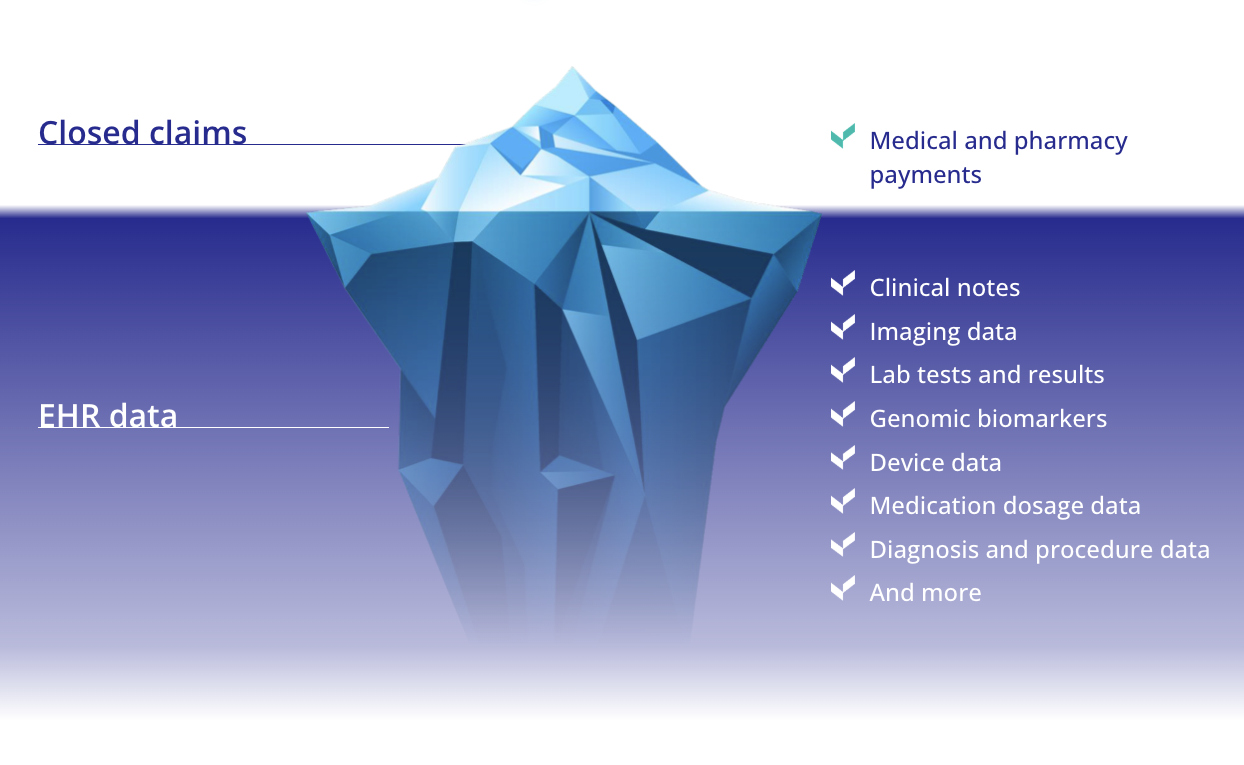
EHR data linked with closed claims provide the complete patient journey
Closed claims
EHR data


Medical and pharmacy payments

Clinical notes

Imaging data

Lab tests and results

Genomic biomarkers

Device data

Medication dosage data

Diagnosis and procedure data

And more

A lifespan of this penguin represents multiple clinical events across care setting from birth to death
EHR linked with closed claims
Closed claims combined with EHR data delivers a longitudinal view of patient journeys providing unprecedented depth and breadth.
EHR linked with closed claims
Closed claims combined with EHR data delivers a longitudinal view of patient journeys providing unprecedented depth and breadth.

Closed claims for 200 million patients from 100+ commercial payers, Medicare, and Medicaid
Closed claims date back to 2016 including medical and pharmacy payments across care setting
All data is accurately linked, expertly de-identified, and immediately available
The Truveta Genome Project will create the largest and most diverse database of genotypic and phenotypic information ever assembled to enable drug discovery, optimize clinical trials, and transform how diseases are prevented, diagnosed, and cured. This genetic data will be linked to de-identified medical records and added to Truveta Data for research.
Daily-updated data cleaned with AI
Billions of data points cleaned with unmatched accuracy
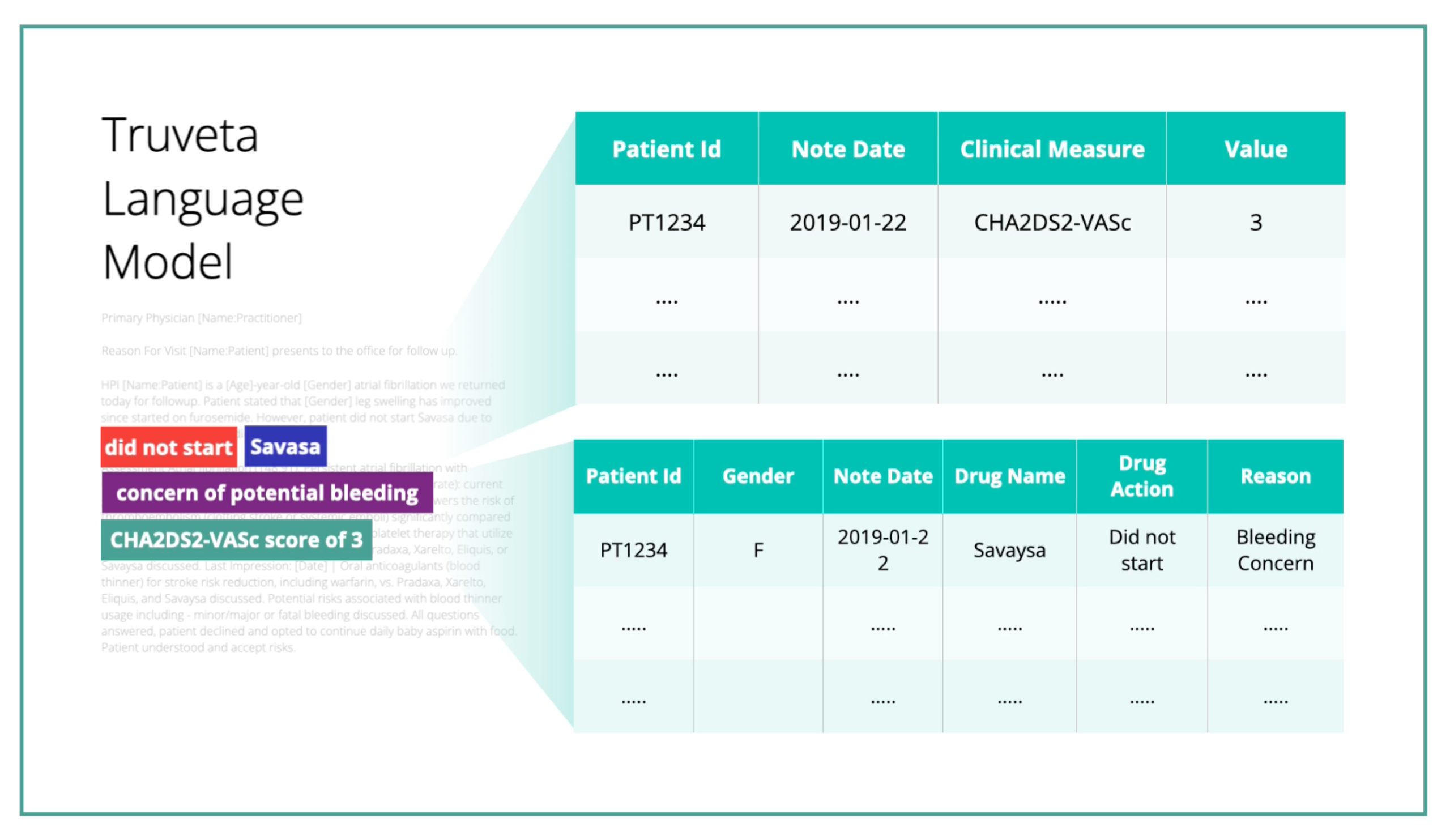
Truveta Language Model, a large-language, multi-modal AI model, transforms billions of data points with industry-leading normalization. Data is carefully de-identified and expertly linked with the highest standards of security and privacy protection.
Explore whitepapers

Truveta Language Model

Data quality

Data security