At the Transcatheter Cardiovascular Therapeutics (TCT) conference in October 2025, Johnson & Johnson MedTech presented two large, contemporary analyses that used Truveta Data to compare Impella microaxial heart pumps with intra-aortic balloon pump (IABP) support in elective, high-risk percutaneous coronary intervention (HR-PCI). Impella is a catheter-based ventricular assist device that supports cardiac output during high-risk procedures; IABP is an established mechanical support option that augments aortic pressure. Together, the TCT presentations report evidence that use of Impella was associated with both improved short-term outcomes and greater cardiac functional recovery, and with lower kidney risk compared with IABP in matched, real-world cohorts.
Study snapshot
Both TCT analyses used Truveta Data, comprised of de-identified electronic health record (EHR) data from US health systems, and focused on non-emergent, high-risk PCI patients. The outcomes and functional-recovery analysis (Impella vs IABP) used de-identified patient records from 2017–2025 and reported results after 1:1 propensity-score matching, producing matched cohorts of about 1,531 patients per arm for main outcomes and a subgroup with LVEF follow-up.
With data from 2015-2023, the renal outcomes study started with larger initial cohorts (Impella ≈4,192; IABP ≈2,314), and then matched patients 1:1 to obtain 766 patients per arm for the kidney analyses. Both teams reported 30- and 90-day outcomes and, where available, longer-term left ventricular ejection fraction (LVEF) recovery up to one year.
Key finding: Impella and heart function
Impella vs IABP in Non-Emergent High-Risk PCI found that patients supported with Impella had lower 30-day mortality and lower acute kidney injury (AKI) rates than matched patients supported with IABP. In the subgroup with follow-up echocardiography, LVEF improved more after Impella support than after IABP support up to 1 year later.
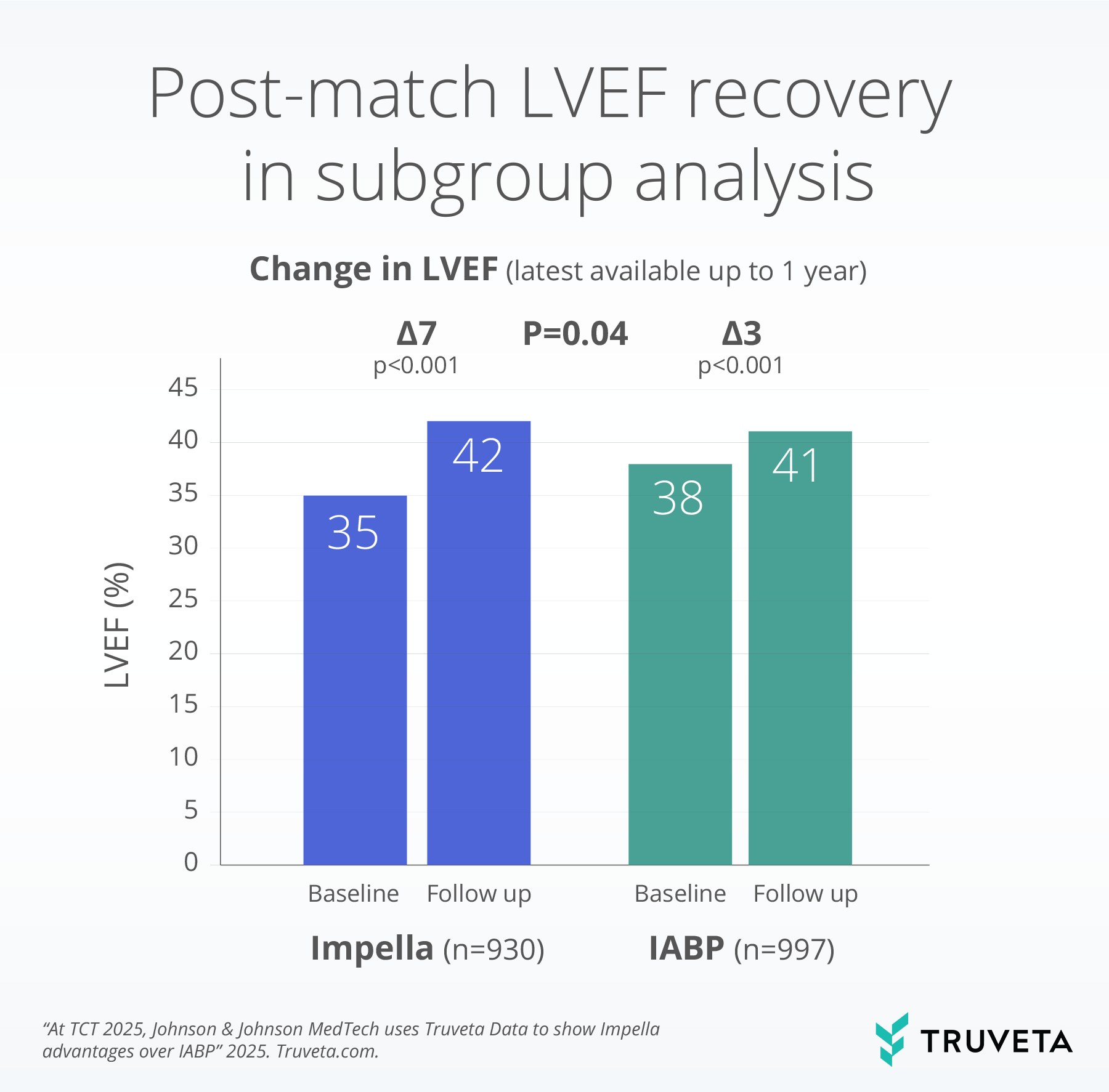
LVEF values are EHR-derived. IABP, intra-aortic balloon pump; LVEF, left ventricular ejection.
Key finding: Impella and kidney outcomes
Renal outcomes after elective high-risk PCI with microaxial flow pump vs. IABP found that Impella (mAFP) use was associated with lower risk of AKI and major adverse kidney events (MAKE) compared with IABP at 30 and 90 days. The study also looked at baseline kidney function and showed the greatest AKI vulnerability in patients with CKD stage 3b—an important clinical subgroup for monitoring and decision-making.
![90-day-outcomes-IABP-vs-mAFP-v2 N=766 vs. 766 for all graphs. [1] New occurrence of kidney failure: any of the following: Diagnosis of N14 (N14.1, n14.11, N14.19). Diagnosis of N17 (including: N17.0, N17.1, N17.2, N17.8, N17.9) acute kidney failure NOT present on admission OR (N19 or N99.0 or T86.12) Diagnosis of kidney failure unspecified NOT present on admission OR (N18.6) Diagnoses of chronic kidney disease end-stage NOT present on admission; [2] MAKE includes mortality OR renal replacement therapy.](/wp-content/uploads/2025/11/90-day-outcomes-IABP-vs-mAFP-v2.png)
Why it matters
Linking device identity and timing to a longitudinal EHR unlocks practical, device-level evidence at scale. In matched, real-world cohorts, Impella support was associated with lower 30-day mortality and AKI and with larger gains in LVEF versus IABP—a three-part signal across outcomes, recovery, and organ-specific safety. For device teams and evidence groups, that kind of auditable, patient-level evidence supports clinical decisions, safety monitoring, and faster answers to evaluate device performance.
Get your questions answered today.
