In this Truveta Experts analysis, Mantas Dmukauskas, PhD examines first-dose HPV vaccination trends using Truveta’s de-identified electronic health record (EHR) data. The analysis tracks annual age-group patterns and sex differences to clarify how changes in recommendations have translated to real-world care.
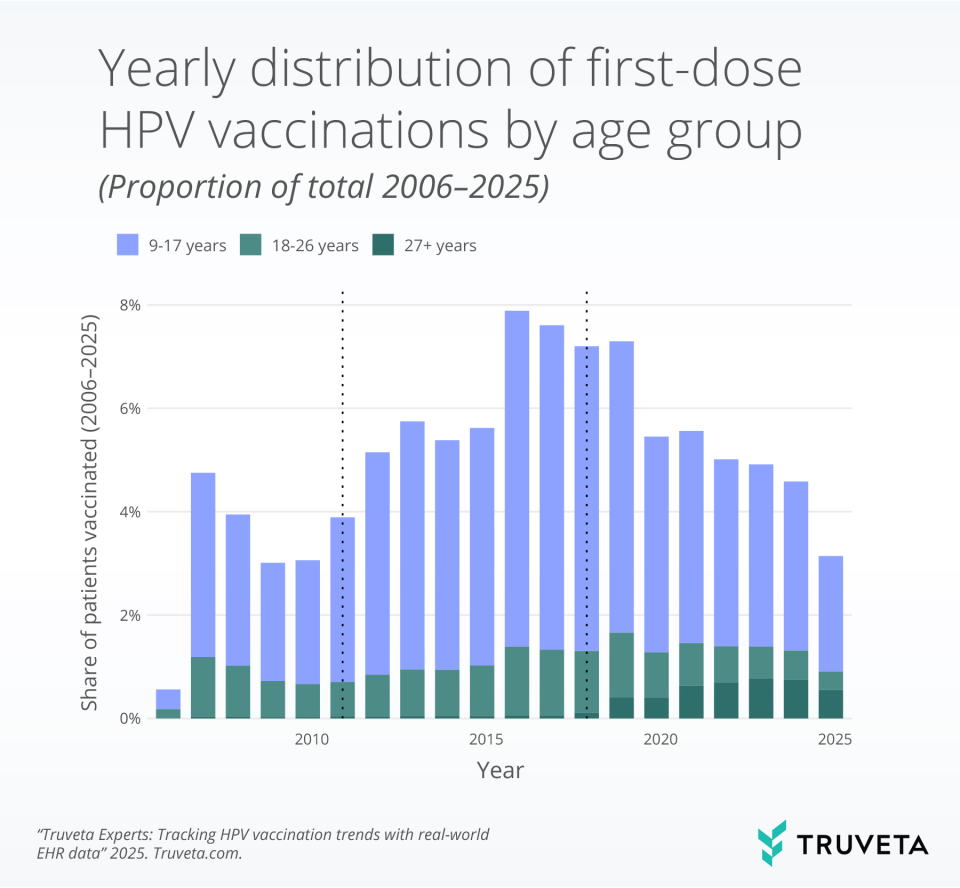
- First-dose HPV initiation fell sharply in 2020 and has not returned to the late-2010s peak, consistent with pandemic disruption of routine adolescent care.
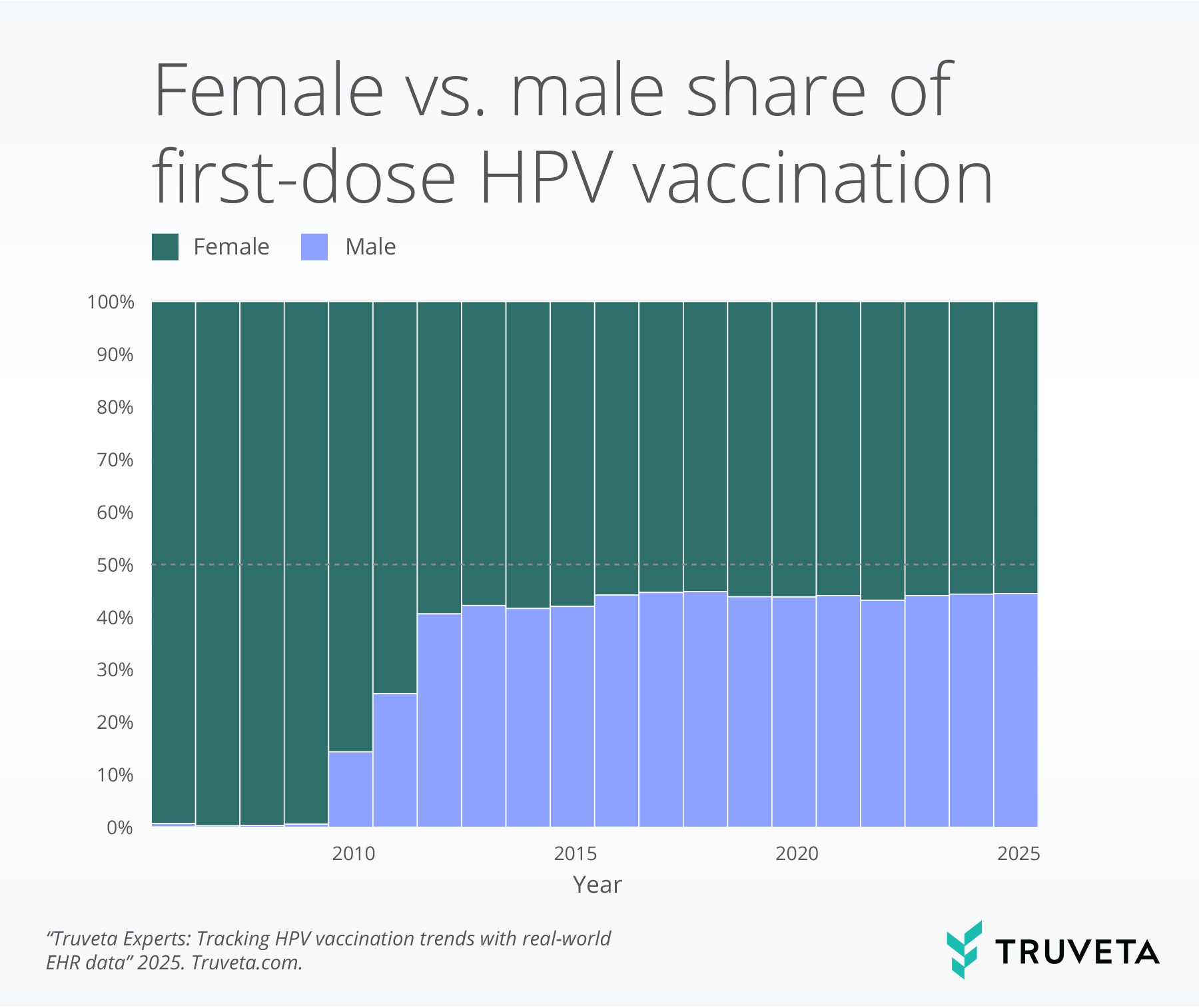
- Male vaccine initiation rose rapidly after boys were added to routine recommendations and reached nearly 45 percent, narrowing the gap between male and female vaccination .
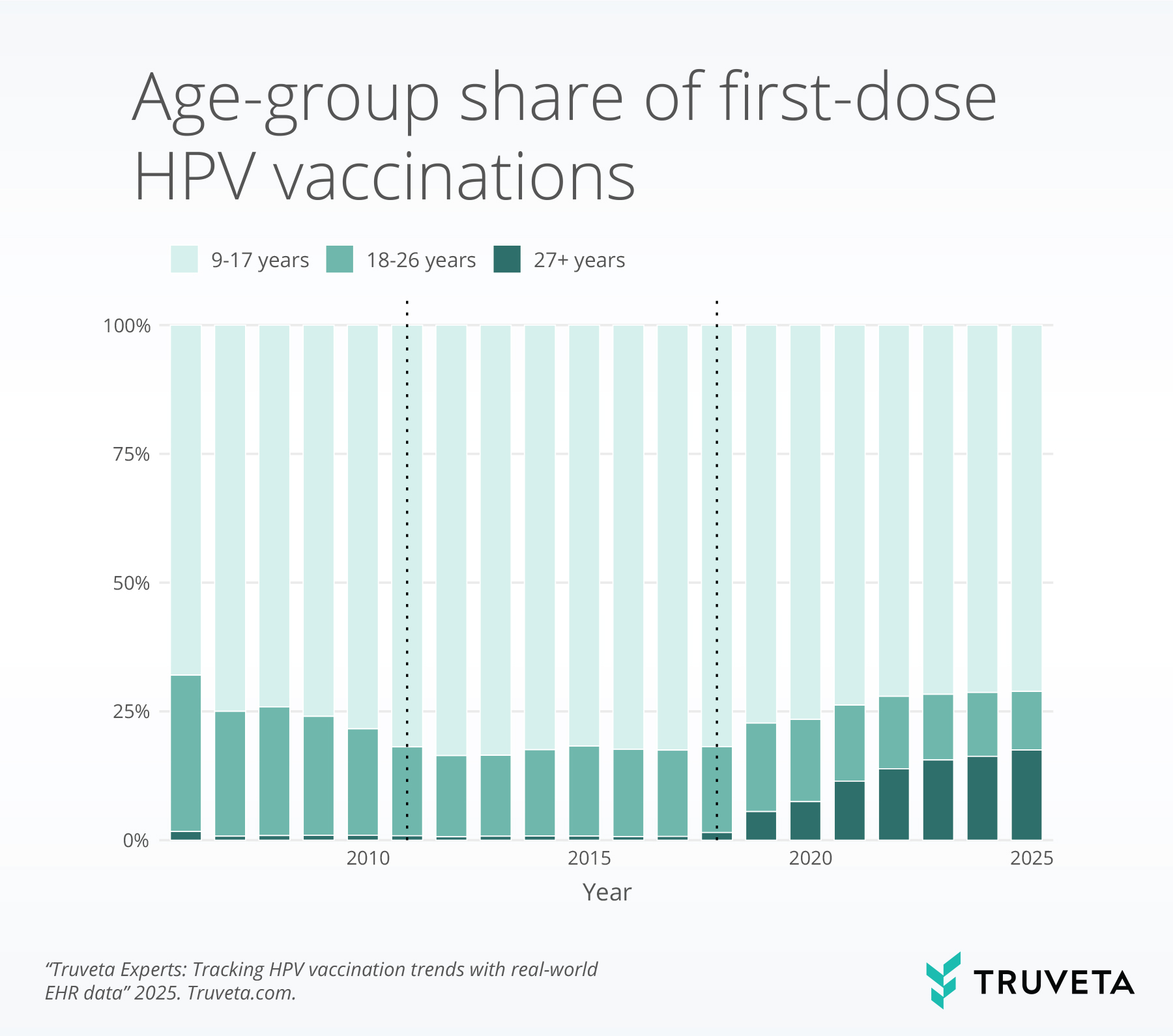
- After 2018, the share of first doses among adults 27+ increased and remains elevated, while adolescents continue to account for roughly three-quarters of first doses.
Human papillomavirus (HPV) causes infections that can progress to cancers across the anogenital tract and the oropharynx (the back of throat, including tonsils and base of tongue). Rates of HPV-related oropharyngeal cancers in men have been rising and now exceed cervical cancer incidence among women in the United States. Preventing HPV infection therefore remains central to cancer prevention strategies. Decades of real-world evidence show strong, lasting protection from HPV vaccination programs, making the HPV vaccine a core element of routine preventive care worldwide.
HPV vaccine recommendations have evolved with the evidence. The vaccine was initially approved and included in routine recommendations for girls in 2006, with recommendations for boys added in 2011. In October 2018, the FDA expanded approval of Gardasil 9 to include men and women aged 27 to 45. Today, vaccination is recommended to start early (around 11-12 years of age). Because vaccination produces the greatest preventive benefit when given in adolescence, monitoring first-dose initiation by age and sex shows whether policy and delivery are protecting those who benefit the most.
These incremental policy changes over the past two decades expanding HPV vaccination eligibility, along with the COVID-19 pandemic, have altered routine vaccination trajectories.
Methods
First-dose HPV vaccine administrations were identified in Truveta’s de-identified electronic health record (EHR) data from 2006–2025 and aggregated by calendar year, sex, and three predefined age groups. We summarized temporal trends using the proportion of all first-dose vaccinations in the study period that occurred in each calendar year (stratified by age).
Yearly demographic distributions were assessed by calculating within-year proportions of vaccinated individuals by sex and age group, with category percentages summing to 100% annually. Analyses compared periods before and after routine HPV vaccine recommendations for boys and expanded adult eligibility and examined the interruption associated with the COVID-19 pandemic in 2020. All figures reflect administered dose captures from member health system EHRs.
Results
The analysis included 2,758,840 first-dose administrations in total: 78.2% (2,156,107) among ages 9–17, 16.9% (467,220) among ages 18–26, 4.8% (131,506) among ages 27 and older, and 0.1% (4,007) among children under 9. The population vaccinated was primarily female 1,748,797 (63.4%).
Annual pattern
First-dose rates rose through the 2000s and peaked in the late 2010s. Rates fell markedly in 2020 and remained below the pre-pandemic high through the end of the observation window, consistent with the documented pandemic disruption to well visits and routine immunizations.
Sex convergence
The proportion of males receiving HPV vaccines increased markedly after routine recommendations for boys were added. From roughly 2010 onward, the proportion of males vaccinated climbed to nearly 45% of the total vaccinated population and have been stable in recent years, producing substantial convergence with female initiation.
Age composition
Adolescents (9–17) account for the majority of first doses, while the share among adults aged 27 and older rose after expanded approvals, shared-decision guidance and remains higher than in earlier years. Because adolescent vaccination delivers the greatest preventive benefit, the shift toward more adult initiation expands reach but does not substitute for adolescent coverage.
Discussion
This analysis points to three related signals: (1) a durable post-pandemic shortfall in first-dose initiation, (2) marked convergence of male and female initiation rates after male routine recommendations, and (3) an increased proporation of adult initiation following eligibility expansion. The findings suggest that restoring adolescent preventive pathways—school-linked programs, standing orders, and reminder/recall systems—should remain a priority, while adult uptake should be treated as complementary to adolescent vaccination.
These findings are consistent with data accessed November 2025. They are preliminary research findings and not peer reviewed; data are constantly changing and updating.
How real-world data enabled this insight
Daily-refreshed, EHR data represening 1 in 3 Americans provide timely visibility into administered first doses across pediatric and adult populations. Truveta’s clinical-level capture of administered immunizations (rather than orders alone), broad geographic coverage, and standardized mappings allow real-time observation of policy effects and the pandemic disruption. The combination of clinical specificity, large sample size, and standardized mappings made it possible to detect policy impacts (expanded recommendations) and the pandemic trough in initiation—signals difficult to assemble from delayed or siloed surveillance systems.
See how Truveta Data can accelerate your next study—Request a customized demo today.
Explore more in this series:
Treatment trends for trigeminal neuralgia
Fireworks during the fourth of July
Real-world trends in Rituxan biosimilar adoption
Sudden cardiac arrest and arrhythmia among patients with chronic kidney disease
Biomarker testing trends in metastatic non-small cell lung cancer

