Why do patients stop taking a medication? Was it due to side effects, access barriers, or lack of efficacy?
These are not just clinical questions; they’re strategic ones. Understanding treatment discontinuation is essential for improving therapy design, informing market access strategies, and differentiating in crowded markets. Unfortunately, most real-world data sources fall short. They either lack information on discontinuation entirely or fail to connect it to clinical context and outcomes.
Why discontinuation matters
High rates of discontinuation are rarely random. They point to real-world gaps in performance, experience, or access. When studied at scale, these reasons become strategic signals:
- Unmet need: Poor efficacy, side effects, or difficult administration point to where current options fall short
- Competitive intelligence: Where and why patients switch away from a therapy can inform positioning and messaging
- Payer strategy: Discontinuation tied to cost, copay, or prior authorization burden highlights opportunity for value frameworks
- Patient engagement: Education and support programs can be better designed when informed by real-world barriers
The most complete real-world data
Discontinuation is not documented cleanly. Structured fields often lack the nuance of why a therapy ended. Claims show gaps or switches, but not what drove them. Even when reasons are recorded in provider notes, most data platforms can’t access or analyze them at scale.
Truveta solves this by providing EHR data—including clinical notes and images—from 30 leading U.S. health systems, representing over 120 million patients. These data are further enhanced with closed claims for 200 million patients, offering the most complete and up-to-date view of therapy use and outcomes available today.
With Truveta, researchers can:
- Extract documented reasons for discontinuation from clinical notes
- Explore treatment timelines, including start, stop, and switching behavior
- Analyze linked outcomes, labs, comorbidities, and SDOH factors
- Integrate closed claims, offering insight into utilization and cost patterns
Scaling insight: GLP-1 study in JAMA Network Open
A recent study from Truveta Research published in JAMA Network Open demonstrates the capability to leverage discontinuation in peer-reviewed research. In the study, “Discontinuation and Reinitiation of Dual-Labeled GLP-1 Receptor Agonists Among US Adults with Overweight or Obesity,” the authors used unstructured notes to extract reasons for discontinuation of GLP-1 therapies in patients with and without diabetes, surfacing real-world tolerability and access challenges that could not be seen in claims or structured EHR data alone.
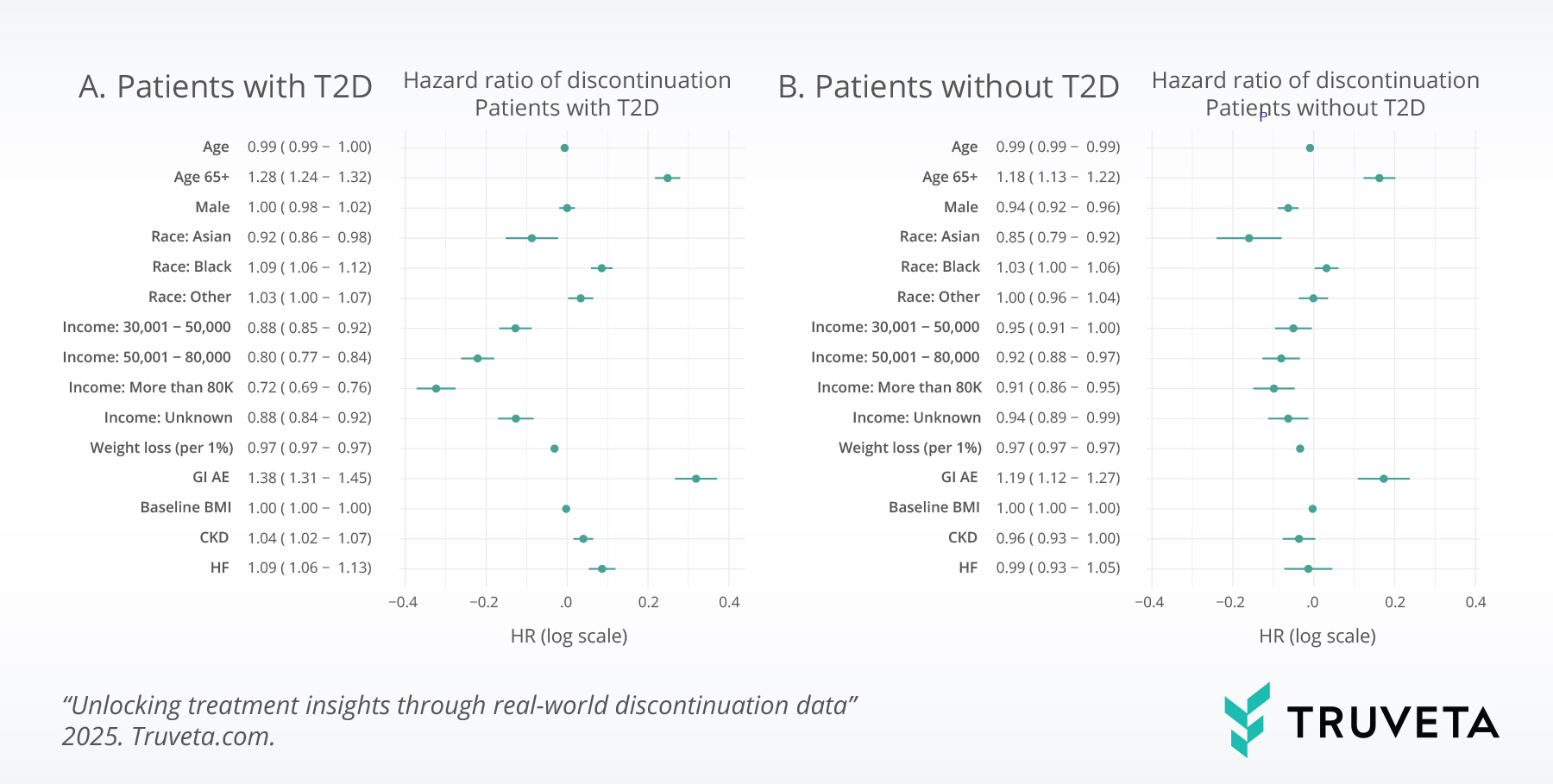
Points represent hazard ratios (HRs) from separate Cox proportional hazards regression models for patients with or without type 2 diabetes, plotted on a log scale. Lines represent 95% CIs, plotted on a log scale. Tabular HRs (95% CIs) are provided on an exponentiated (HR) scale. The comparator reference level for sex was female, for race and ethnicity was White, and for income was $30 000 or less. Baseline hazards were stratified by initiation year. BMI indicates body mass index; CKD, chronic kidney disease.
Data spotlight: Atopic dermatitis
To further illustrate the power of these data, Truveta recently conducted a feasibility analysis in atopic dermatitis (AD)—a chronic, relapsing condition with growing global prevalence and a competitive systemic therapy market. The fourth leading cause of non-fatal disability, AD is also associated with asthma, allergic rhinitis, anxiety/depression, and other immune-mediated diseases, spurring high demand for systemic treatments.
Truveta identified:
- 968,000+ patients with AD identified using EHR data
- 41% of identified patients have linked closed claims to support robust health economics research
- Biologic and JAK inhibitor use measurable across tens of thousands of patients
- Documented reasons for discontinuation including cost, drug failure, and adverse reaction
- Clinical outcomes available in notes, offering longitudinal context beyond fill rates
Turning data into direction
Understanding why patients stop therapy is just as important as knowing when they start. Discontinuation insights offer a window into unmet need, barriers to access, and real-world outcomes that shape every stage of the product lifecycle.
By combining the daily-update EHR data from more than 120 million patients, including clinical notes and images, linked with closed claims of 200 million patients, Truveta delivers the most complete, timely, and representative data required to study discontinuation with precision.
Bring your questions to life
Interested in exploring discontinuation insights? Truveta’s team of data scientists, epidemiologists, and clinicians can help uncover the why.



