Section of Cardiovascular Medicine, Department of Internal Medicine, Yale School of Medicine, New Haven, CT, , Mitsuaki Sawano, MD, PhD ⊕Center for Outcomes Research and Evaluation, Yale New Haven Hospital, New Haven, CT
Section of Cardiovascular Medicine, Department of Internal Medicine, Yale School of Medicine, New Haven, CT, Patricia J. Rodriguez, PhD, MPH ⊕Truveta, Inc, Bellevue, WA
Brianna M. Goodwin Cartwright, MS ⊕Truveta, Inc, Bellevue, WA, Leslie H. Curtis, PhD ⊕Departments of Population Health Sciences and Medicine, Duke University School of Medicine, Durham, NC, Nicholas L. Stucky, MD, PhD ⊕Truveta, Inc, Bellevue, WA
- The FDA’s expanded indication to include cardiovascular disease for anti-obesity medication (AOM) semaglutide in March 2024 was associated with a shift in GLP-1 AOM first-time prescribing and dispensing patterns.
- In the month immediately after the approval, there was a 50% increase in first-time AOM semaglutide prescribing and dispensing for people with overweight or obesity and cardiovascular disease.
- Despite the eligibility of many patients with overweight or obesity and cardiovascular disease for GLP-1 receptor agonists, the overall rate of first-time prescribing and dispensing of AOM semaglutide remained low, which represents an opportunity to improve cardiovascular outcomes.
There are multiple glucagon-like peptide-1 receptor agonists (GLP-1 RAs) with label indications for prevention of obesity and type 2 diabetes. In March 2024, the FDA approved anti-obesity medication (AOM) semaglutide for reduction of the risk of serious heart problems like heart attack, stroke, and cardiovascular death in adults who have both heart disease (CVD) and are overweight or obese (1). AOM semaglutide is the first GLP-1 RA to receive this expanded label for heart-protective benefits.
Although the label has been expanded for over a year, there is little knowledge about changes in prescribing and dispensing of AOM semaglutide for a population with cardiovascular disease. In this analysis, we aimed to understand the rate of new cases of AOM GLP-1 RA prescribing and dispensing among US adults with both heart disease and excess weight between January 2021 and October 2024.
Methods
We identified patients who had a first-time prescription or prescription fill of three AOM GLP-1 RAs (semaglutide, tirzepatide, and liraglutide). GLP-1 RAs were identified by the brand names and standard codes (RxNorm and NDC codes). Although tirzepatide and liraglutide did not receive an expanded label indication, they were included in this analysis as a reference.
Unadjusted rates
Impact of the FDA approval
We adjusted for age (categories: 18-34, 35-49, 50-64, and 65+ years), sex, race (Asian, Black, White, other race, and unknown), Hispanic ethnicity, and BMI category (overweight: BMI 27-29.9, obesity class 1: BMI 30-34.9, obesity class 2: BMI 35-39.9, and obesity class 3: BMI 40 or greater).
Poisson regression model was used to assess the association between the FDA’s approval for new indication and incidence of prescribing/dispensing of AOM semaglutide. We reported the outputs of the interrupted time series model.
Results
Unadjusted rates
We did not observe any significant changes in first-time prescribing and dispensing rates of AOM liraglutide during the study period.
However, the incidence rates of first-time prescribing and dispensing of the three GLP-1 receptor agonists (liraglutide, semaglutide, and tirzepatide) overall was low across the observed period for the patient population eligible for GLP-1 treatment for cardiovascular disease and overweight or obesity. For instance, even after the FDA’s indication expansion for AOM semaglutide, fewer than 100 per 100,000 patients eligible for this medication actually filled the medication for the first time at a pharmacy.
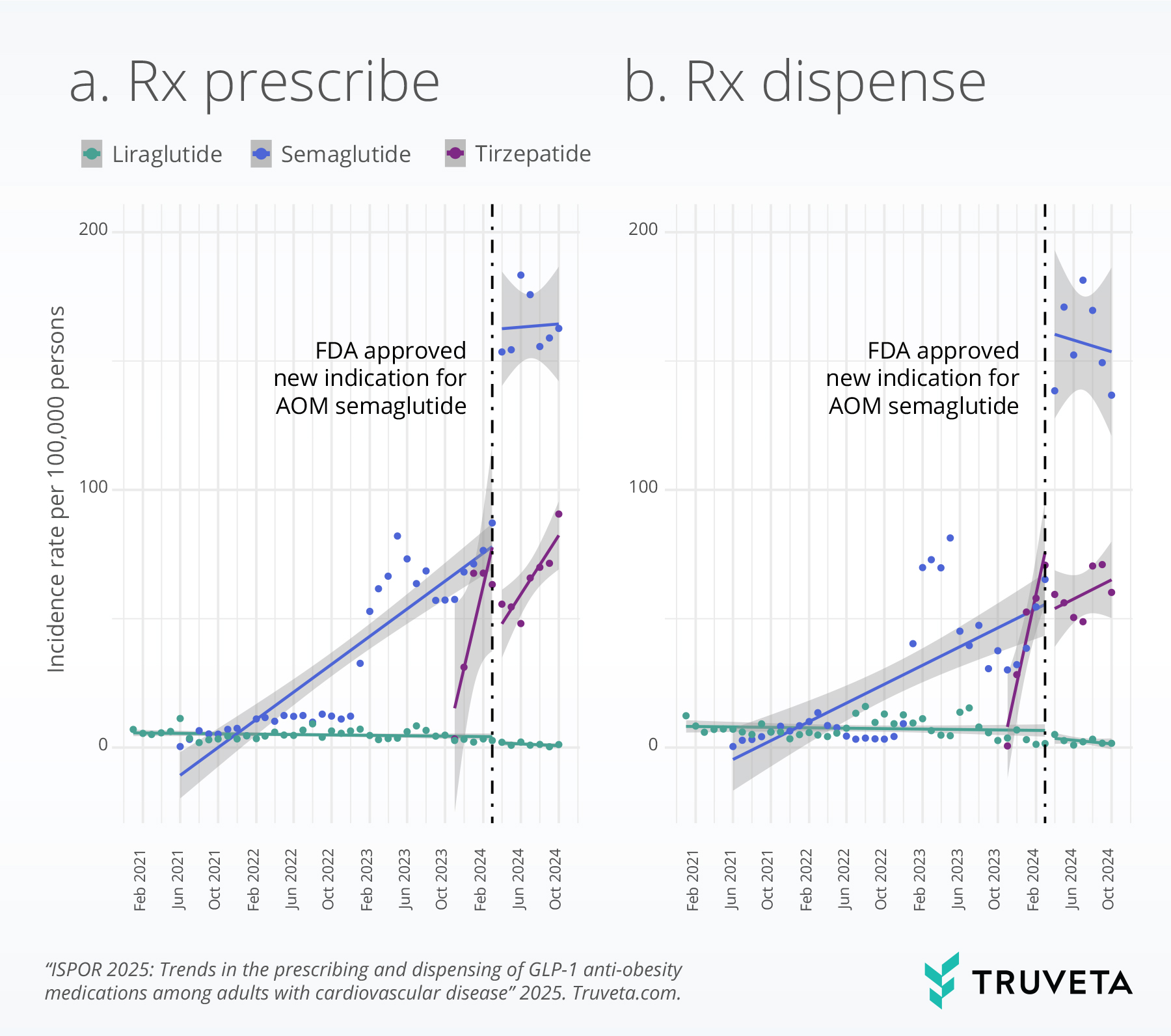
Interrupted time series analysis
This method allowed us to examine changes in first-time prescribing and dispensing rates associated with this regulatory event, while accounting for potential confounding factors including age, sex, race, ethnicity, and body mass index (BMI).
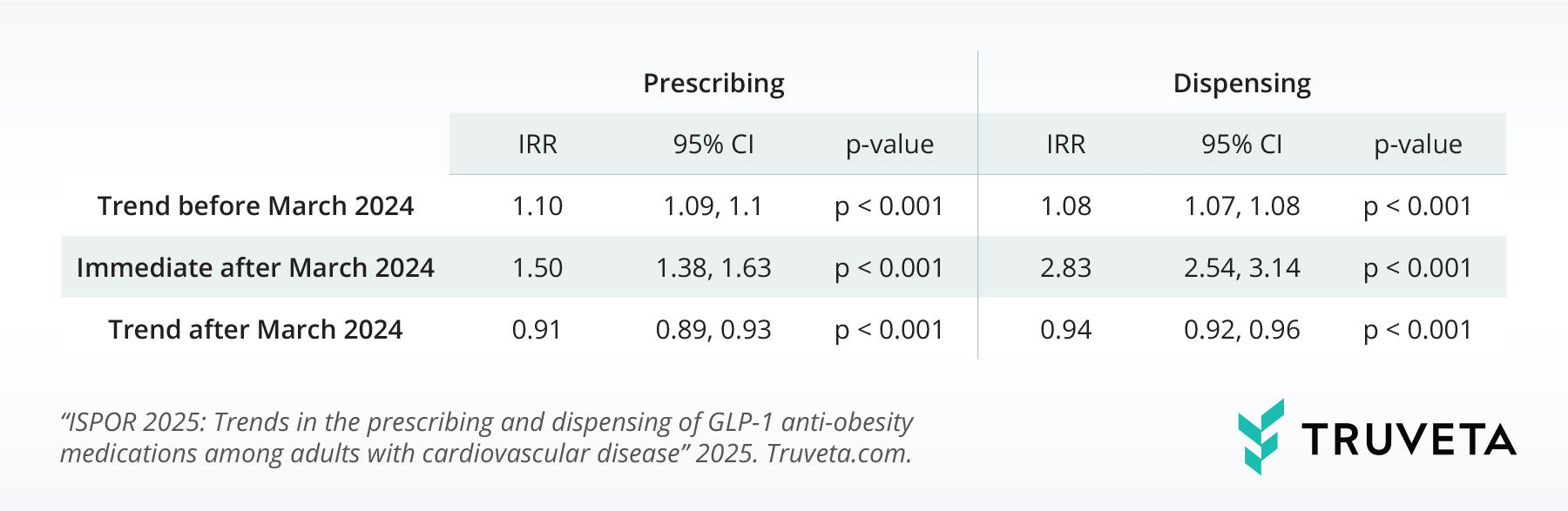
In the month immediately after the approval, the rate at which AOM semaglutide was prescribed increased by 50% (95% CI, 38% – 63%). This indicates a rapid adoption of the medication for its newly approved cardiovascular benefits. Even more striking was the change in first-time dispensing rates. The rate of first-time AOM semaglutide prescriptions filled by patients jumped by an impressive 183% (95% CI, 154% – 214%) compared to the levels seen just prior to the FDA’s announcement. This substantial increase underscores a swift response from both prescribers and patients to the expanded therapeutic applications of AOM semaglutide.
However, when considering the pre-approval trends, the rate of growth in both first-time prescribing and dispensing of AOM semaglutide slowed down after the indication expansion. Specifically, the monthly rate of first-time prescribing decreased by 9% (95% CI: -11% to -7%), and the monthly rate of first-time dispensing decreased by 6% (95% CI: -8% to -4%) relative to the pre-approval trajectory.
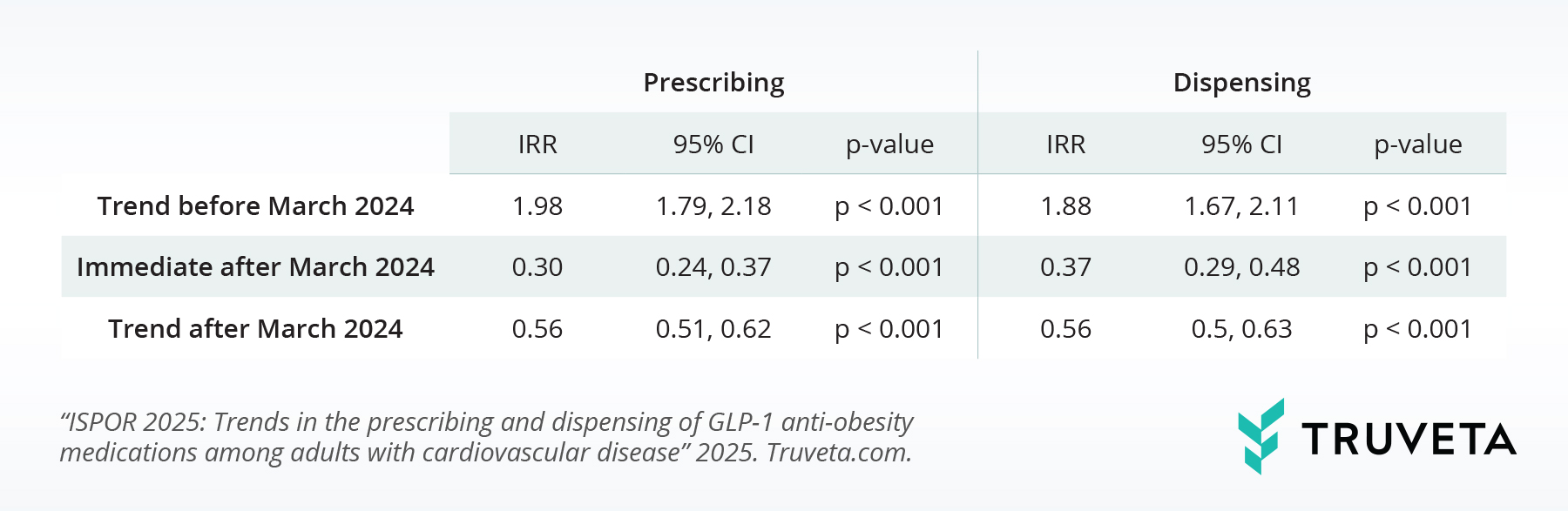
Table 2 explores how AOM semaglutide’s new approval affected first-time AOM tirzepatide use. Before the approval, first-time AOM tirzepatide prescribing rose about 98% monthly 95% CI, 79% – 118%), and first-time dispensing increased around 88% monthly (IRR, 1.88; 95% CI, 67% – 111%), showing strong pre-approval growth.
Discussion
Despite the eligibility of many patients with cardiovascular disease and overweight or obesity for GLP-1 receptor agonists, the overall incidence of first-time prescribing and dispensing of liraglutide, semaglutide, and tirzepatide was low across the board during the study period. This suggests a potential gap in the utilization of these guideline-recommended therapies within this population.
Interestingly, after the FDA’s approval of AOM semaglutide for heart protection in March 2024, we observed a clear pattern. Initially, both the prescribing and dispensing of first-time AOM semaglutide increased substantially. However, this surge was followed by a decrease or leveling off. In contrast, the first-time prescribing and dispensing of AOM tirzepatide saw a sharp decline initially, but then started to increase again. We didn’t see any major changes in the use of liraglutide.
The initial excitement and subsequent leveling off in first-time AOM semaglutide prescribing and dispensing highlights the complicated interplay of factors like insurance coverage, medication costs, and medical recommendations – among others – in shaping how treatments are adopted over time. This kind of data-driven analysis, made possible with resources like Truveta, is crucial for understanding the long-term effects of new medical approvals and changes in healthcare policies on who gets access to these treatments and what their health outcomes are.
Data were accessed on February 8, 2025.
Citation
- U.S. Food and Drug Administration. (2024, March 8). FDA approves first treatment to reduce risk of serious heart problems specifically in adults with obesity or overweight. [FDA]. Retrieved from https://www.fda.gov/news-events/press-announcements/fda-approves-first-treatment-reduce-risk-serious-heart-problems-specifically-adults-obesity-or


