- AOM tirzepatide prescriptions rates, which had been rising by an average of +0.06 percentage points per month (Jan 2024–Jun 2025), grew only +0.03 points from June to July 2025 after the CVS GLP-1 formulary change. In contrast, AOM semaglutide increased by +0.1 points from June to July—its largest one-month gain in the study period, compared with an average of just +0.02 points per month previously.
- From January 2024–June 2025, AOM tirzepatide initiations rose steadily (+239% cumulative increase, ~+0.01 points/month). But between June and July 2025, AOM tirzepatide initiations declined slightly, marking the first monthly drop in nearly a year. In contrast, first-time prescribing of AOM semaglutide, which had been declining (~–0.004 points/month), rose by +4.9% from June to July 2025—its strongest monthly increase in over a year.
Limited data exists on prescribing patterns for patients receiving GLP-1 RAs. Therefore, each quarter, Truveta Research provides a report describing first-time and overall prescribing of GLP-1 RA drugs. Our most recent report—with data through June 2025—showed increases in overall anti-obesity medication (AOM) prescribing and AOM tirzepatide and semaglutide compared to March 2025 (1).
CVS Caremark announced on May 1, 2025 that AOM tirzepatide (sold as Zepbound) would be removed from its standard preferred formulary effective July 1, 2025 (2, 3). In its place, AOM semaglutide (sold as Wegovy) was designated as the preferred therapy for obesity management. Importantly, the decision applies only to the obesity indication—tirzepatide remains available in its anti-diabetic medication (ADM) formulation (sold as Mounjaro) for patients with type 2 diabetes. CVS Caremark manages prescription benefits for more than 25-30 million Americans (4), meaning this formulary change could directly affect tens of millions of people. Patients currently using AOM tirzepatide may need to switch to AOM semaglutide to maintain insurance coverage of the medication, while those wishing to remain on tirzepatide may face higher out-of-pocket costs or coverage denials.
In this analysis, we conducted an analysis to understand the potential impacts of the CVS formulary change on prescribing patterns. In parallel, we released a companion report that uses dispense data to examine switching patterns during the same period as the CVS formulary change, providing a complementary perspective on how patients transitioned between GLP-1 RAs.
Methods
Using a subset of Truveta Data, we identified adults who were prescribed a GLP-1 RA medication between January 01, 2024 and July 31, 2025. Medications included semaglutide (AOM and ADM, oral and injectable), liraglutide (AOM and ADM), exenatide (ADM), lixisenatide (ADM), tirzepatide (AOM and ADM), and dulaglutide (ADM). Although our primary analysis focused on AOM tirzepatide and AOM semaglutide, we extracted the prescription data for all GLP-1 RAs. Brand names were used to classify medications by labeled indication (AOM vs. ADM) and route of administration (oral vs. injectable); medications were excluded if brand name information was unavailable.
We describe first-time and overall prescribing rates compared to all prescriptions.
Results
We included 1,647,238 patients who were prescribed a GLP-1 RA between January 2024 and July 2025, with 6,059,445 total GLP-1 RA prescriptions during this period.
Overall prescribing patterns
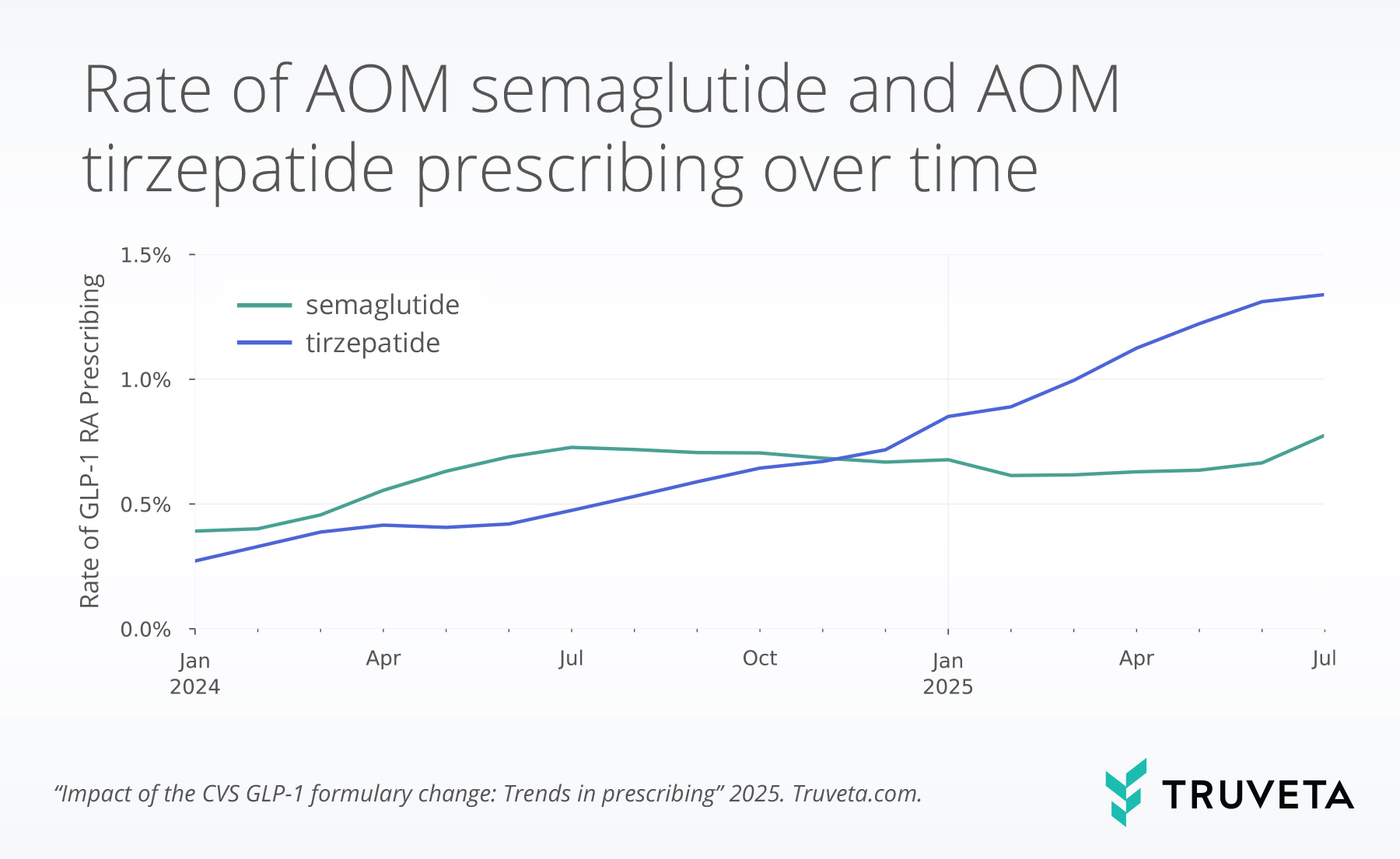
Prescribing of AOM medications has evolved over the past 18 months, with observed shifts between AOM semaglutide and AOM tirzepatide. In early 2024, AOM semaglutide prescriptions were steadily increasing; in January 2024, they accounted for 0.4% of all prescriptions and increased to 0.7% by July 2024. However, prescribing rates remained relatively steady and even decreased slightly through late 2024 and early 2025. AOM tirzepatide, in contrast, began lower at 0.3% in January 2024, but grew rapidly, surpassing prescribing rates of AOM semaglutide by late 2024. By May 2025, prescribing reached 1.2% of all prescriptions, nearly double AOM semaglutide’s prescribing rates.
However, in the last month, following the CVS formulary change, this trajectory shifted again. Instead of continuing its rapid climb, AOM tirzepatide growth slowed considerably. AOM tirzepatide prescription rates increased by an average of 0.06 percentage points a month from January 2024 to June 2025 but grew only by 0.03 percentage points between June and July.
At the same time, prescription rates for AOM semaglutide increased by 0.1 percentage points from June to July, compared with the average monthly increase of 0.02 percentage points between January 2024 and June 2025. This June to July increase was semaglutide’s largest month-to-month gain during the study period.
First-time prescribing
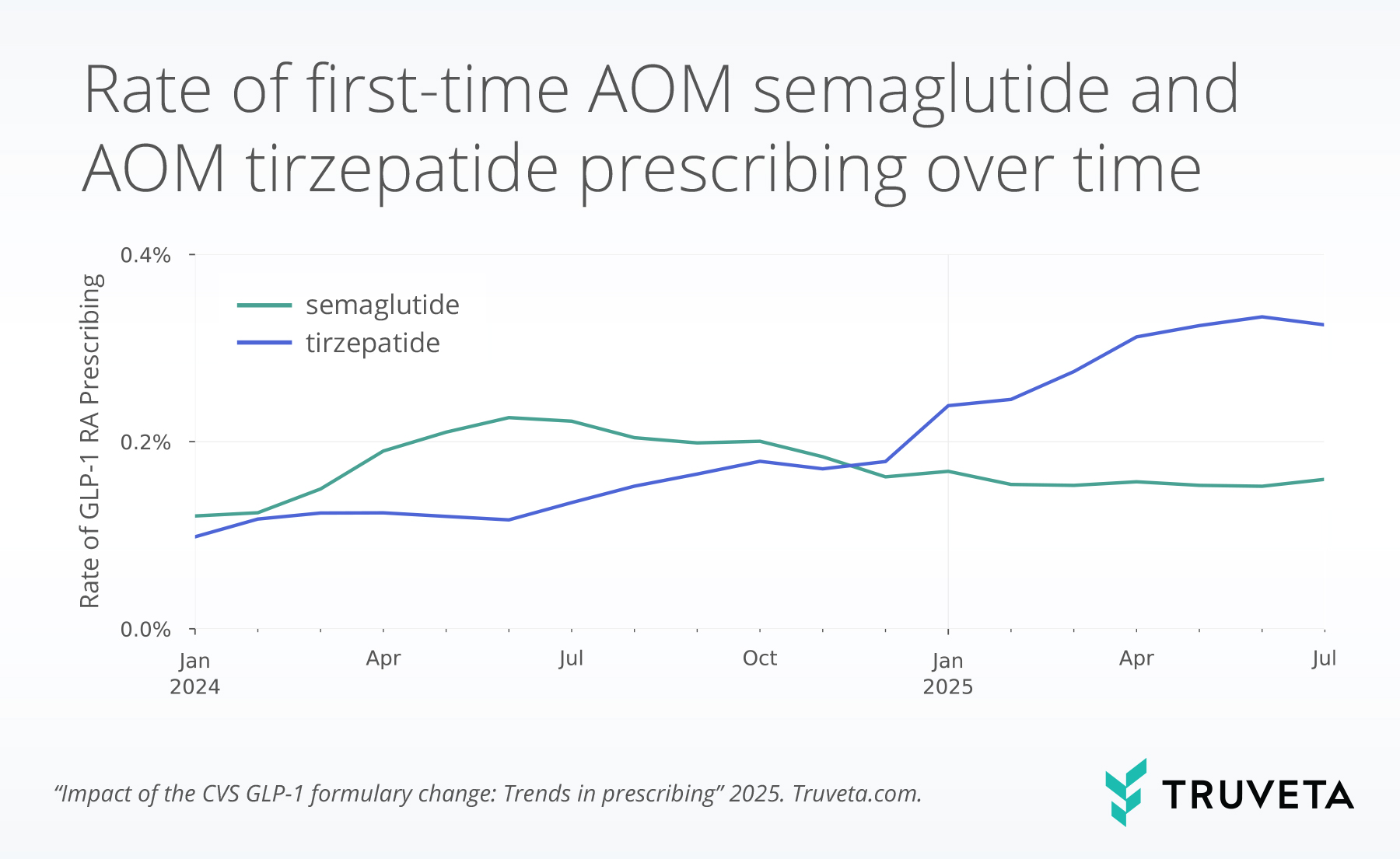
First-time prescribing of AOM medications show similar trends to overall use. First-time prescribing of AOM tirzepatide has climbed steadily from early 2024 through mid-2025. Between January 2024 and June 2025, first-time prescribing increased at an average rate of 0.01 percentage points per month, a 239% gain over the period. From June to July, however, AOM tirzepatide declined slightly, marking the first month without continued first-time prescriptions growth in nearly a year. First-time prescriptions of AOM tirzepatide also did not increase from November to December 2024, likely due to the holidays.
First-time prescribing rates of AOM semaglutide increased in early 2024 and then have remained relatively stable and flat. The first-time AOM semaglutide prescribing rate was 0.1% in January 2024 and reached 0.2% by July 2024. However, over the next year, initiation slowed and even reversed: with first-time prescribing rates declining 31% by June 2025, reflecting an average month-over-month decrease of 0.004 percentage points. Yet in the most recent month, from June to July 2025, AOM semaglutide prescribing increased by 4.9%, the largest one-month gain in first-time AOM semaglutide prescribing in over a year.
Discussion
Between June and July 2025, overall prescribing rates increased for both AOM semaglutide and AOM tirzepatide; however, the rise was more pronounced for AOM semaglutide. This divergence coincides with the CVS formulary change and is further reflected in first-time prescribing patterns. Initiations of AOM semaglutide rose between June and July, while first-time prescribing of AOM tirzepatide declined. This is the first time initiation of AOM semaglutide increased and initiation of AOM tirzepatide decreased in over a year. This suggests that the formulary change may have not only increased AOM semaglutide prescriptions but also increased first-time use of AOM semaglutide.
These prescribing patterns show how payor decisions can rapidly reshape the GLP-1 landscape. While AOM tirzepatide has higher rates of prescriptions, its momentum slowed after the CVS formulary shift, just as AOM semaglutide shows potential renewed growth. This dynamic illustrates the sensitivity of prescribing trends to access and coverage changes, even for high-demand medications. The CVS Caremark formulary change provides a real-world case study in how payer decisions shape treatment trajectories for patients with obesity. Prescribing patterns may provide early insight into how physicians and patients anticipate changing cost dynamics, even before those changes are fully realized at the pharmacy counter. It’s also important to understand how these changes affect which medications patients receive at the pharmacy, and we’ve released a companion report that uses dispense data to examine switching patterns.
Several limitations warrant consideration. First, these data reflect prescribing patterns only through July 2025 and may not capture whether the trends persist over a longer period following the formulary change. Second, prescribing does not necessarily equate to dispensing or treatment initiation. Some patients may have received a prescription but ultimately were unable to fill it due to insurance coverage, cost-sharing requirements, or pharmacy-level barriers. Finally, the observed changes in prescribing behavior occurred at the same time as the CVS Caremark formulary change; however, this analysis did not evaluate causality. We did not include an analysis of patient payor; and therefore, we cannot confirm that the CVS formulary change directly caused the shift in prescription trends. Other factors—such as supply dynamics, regional payor policies, or provider preferences—may also have contributed.
These are preliminary research findings and not peer reviewed. Data are constantly changing and updating. These findings are consistent with data accessed on August 25, 2025.
Citations
- S. Gratzl, B. M. G. Cartwright, P. J. Rodriguez, K. Gilbert, D. Do, N. Masters, N. L. Stucky, Monitoring Report: GLP-1 RA Prescribing Trends – June 2025 Data. [Preprint] (2025). https://doi.org/10.1101/2025.03.06.25323524.
- CVS Health, Improving access and affordability to high-cost weight management drugs (2025). https://www.cvshealth.com/news/pbm/improving-access-and-affordability-to-high-cost-weight-management-drugs.html.
- Fraiser Kansteiner, In major blow for Lilly, Novo strikes deal giving Wegovy preferred access on CVS Caremark formulary (2025). https://www.fiercepharma.com/pharma/major-blow-lilly-novo-strikes-deal-giving-wegovy-preferred-access-cvs-caremark-formulary.
- A. Bratulic, Lilly’s Q1 revenue surge overshadowed by CVS dumping Zepbound for Wegovy | FirstWord Pharma, FirstWord Pharma (2025). https://firstwordpharma.com/story/5955788.


