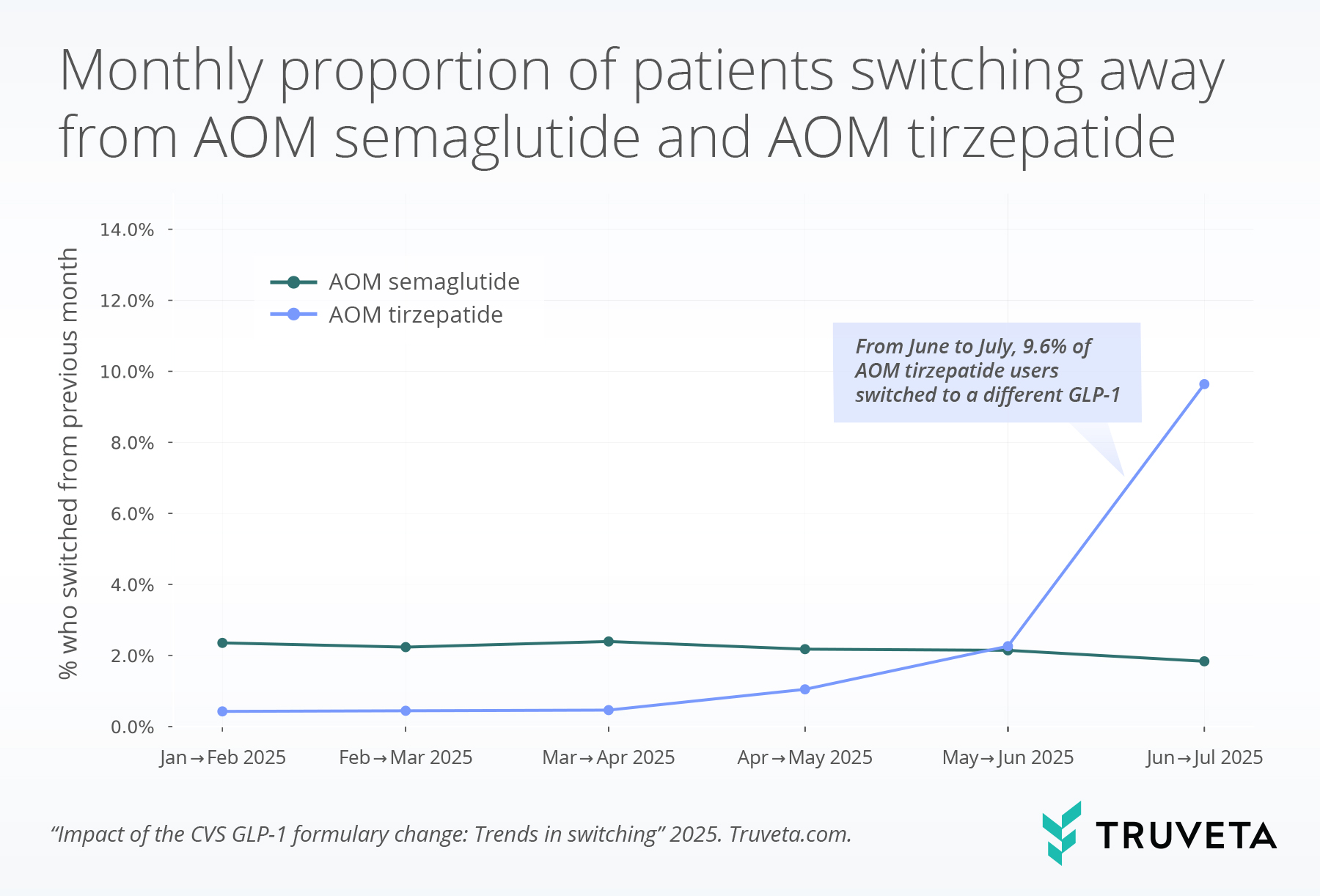
- In July 2025, following the CVS formulary change, 9.6% of patients on AOM tirzepatide switched to a different drug. This was more than a 16-fold increase compared with the average earlier in the year.
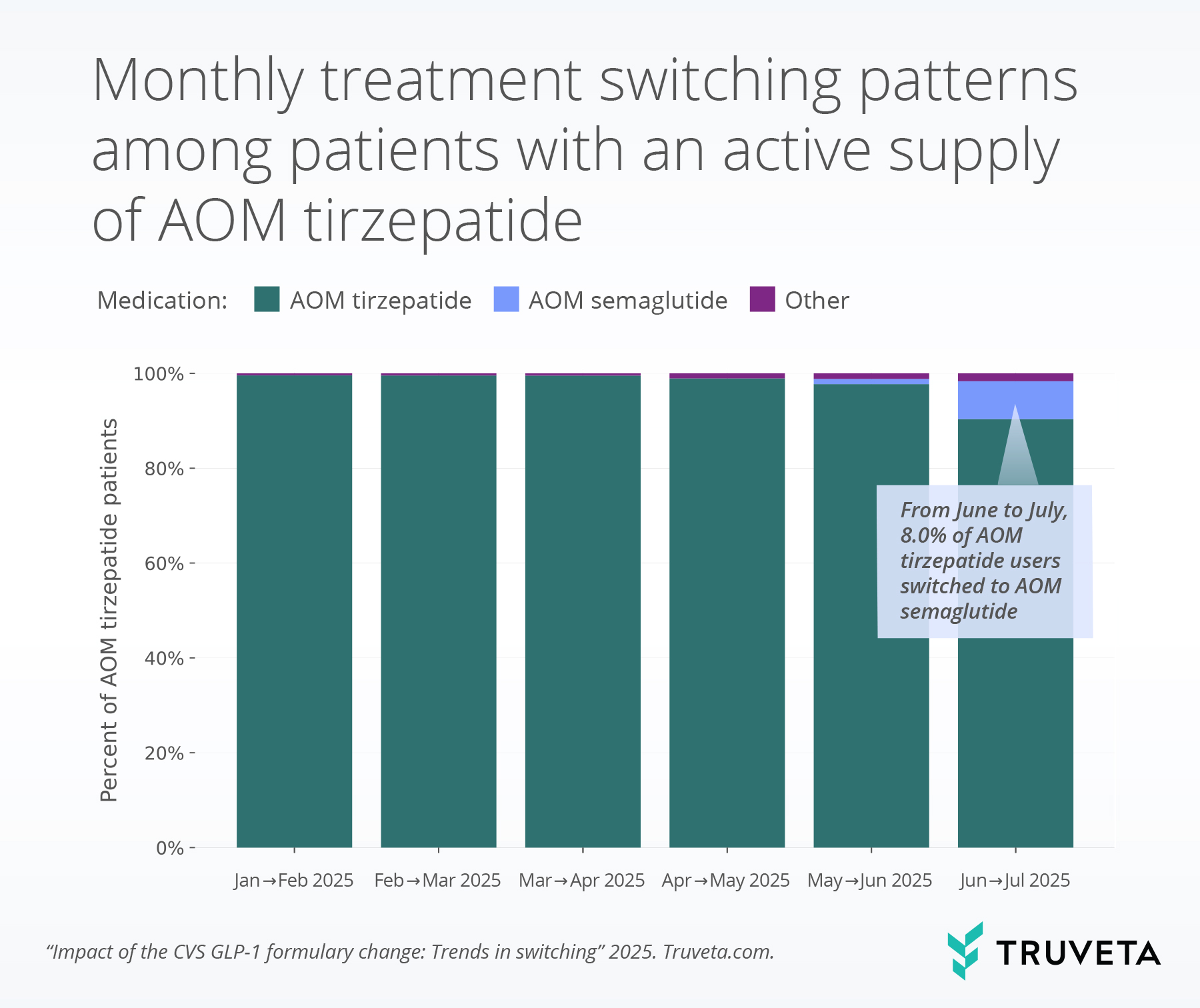
- Among AOM tirzepatide users who switched to a different GLP-1 medication between June and July, 82.8% (more than 8 out of 10) moved to AOM semaglutide.
GLP-1 RAs are one of the most significant therapeutic advances in the management of type 2 diabetes and obesity over the past decade (1, 2). Their demonstrated effectiveness in improving glucose control, supporting weight loss, and cardiovascular benefits has made them some of the most widely prescribed medicines (1, 3–5). Our recent analysis has shown this class of drugs accounts for over 6% of all prescriptions and an estimated 12% of adults have taken a GLP-1 RA medication (6, 7). In real-world settings, access to GLP-1 RAs is shaped by practical considerations, such as drug availability, insurance coverage, and formulary decisions that can directly impact the treatments millions of patients receive.
The dynamic nature of anti-obesity (AOM) GLP-1 RA coverage is illustrated by CVS Caremark’s formulary change, announced on May 1, 2025 (8). Effective July 1, 2025, AOM tirzepatide (sold as Zepbound) was removed from its standard preferred formulary (9). In its place, AOM semaglutide (sold as Wegovy) was designated as the preferred therapy for obesity management. Importantly, this decision applied only to the obesity indication—tirzepatide remains available in its anti-diabetic medication (ADM) formulation (sold as Mounjaro) for patients with type 2 diabetes.
We have previously examined GLP-1 RA switching between January 2018 and October 2023, finding that switching patterns varied across medications and time but often reflected the impact of drug supply shortages. Building on that work, we wanted to understand how the recent CVS formulary change would impact patients’ ability to receive AOM semaglutide and/or AOM tirzepatide. In parallel, we also released a companion report looking at GLP-1 prescription data to establish a current view of utilization trends.
Methods
We used a subset of Truveta Data to identify patients aged 18 years or older with overweight or obesity (defined as BMI over 27) and at least one medication dispense (i.e., prescription filled at a pharmacy) for a GLP-1 RA between January 2025 and July 2025. Medications included semaglutide (AOM and ADM, oral and injectable), liraglutide (AOM and ADM), exenatide (ADM), lixisenatide (ADM), tirzepatide (AOM and ADM), and dulaglutide (ADM). Although our primary analyses focused on AOM tirzepatide and AOM semaglutide, we extracted dispense data for all GLP-1 RAs to examine overall treatment transitions and identify which therapies patients switched to. Brand names were used to classify medications by labeled indication (AOM vs. ADM) and route of administration (oral vs. injectable); medications were excluded if brand name information was unavailable.
Dispense data are prescriptions filled both within and outside Truveta’s health system members, allowing broader observability of medication history. Dispense histories are updated at each encounter and include fill dates. For prescription fills exceeding a 30 day supply, we assumed supply extended across subsequent months (e.g., a 60-day fill was counted as two months of supply); henceforth referred to as an ‘active supply.’ All patients were required to have at least two consecutive-months of GLP-1 RA active supply during the study period. Switching was defined as a patient with an active supply of a different GLP-1 RA in two sequential months.
We performed two analyses looking at switching patterns across consecutive months (e.g., Jan→Feb 2025). First, for patients with an active supply of AOM tirzepatide and AOM semaglutide, we calculated the percentage who switched to a different GLP-1 RA. Patients could contribute to multiple month pairs if they had an active supply in multiple months. This approach assessed persistence versus switching among those active in both months, and therefore, did not evaluate discontinuation (no fill in the subsequent month). Second, for patients with an active supply of AOM tirzepatide, we calculated the monthly distribution of GLP-1 RA use in the subsequent month, which includes both those who remained on the same therapy and those who switched to a new drug.
Results
Population
The study population included 609,406 patients. About half of patients were between the ages of 45 and 60 (52.2%), nearly two-thirds were female (62.2%), and most identified as white (70.9%) and non-Hispanic or Latino (82.9%). More than half of patients (54.8%) had type 2 diabetes.
Across all GLP-1 RAs studied, 6.6% of patients switched to a different drug at least once in a subsequent month during January–July 2025.
Switching away from AOM semaglutide and AOM tirzepatide
Prior to May 2025, patients with an active supply of AOM semaglutide or AOM tirzepatide during a given month rarely switched to a different drug. From January to April 2025, the average monthly switching rate to a different drug was 0.6% for AOM tirzepatide and 2.3% for AOM semaglutide.
Among patients with an active supply of AOM tirzepatide in June, 9.6% switched to a different drug in July, representing a 16-fold increase in switching compared with the period before the formulary change announcement.
In contrast, switching among patients with an active supply of AOM semaglutide declined from June to July.
Switching patterns among patients with AOM tirzepatide
Between January and April, 99.4% of patients with an active supply of AOM tirzepatide in a given month had an active supply of AOM tirzepatide in the subsequent month, showing strong persistence with very limited switching.
However, in June we observed a shift: among patients with an active supply of AOM tirzepatide, 90.4% had an active supply of the AOM tirzepatide in July, while 8.0% switched to AOM semaglutide. A small proportion of patients (0.9%) switched to ADM tirzepatide. Among those who switched, more than 8 out of 10 (82.8%) patients switched to AOM semaglutide.
Discussion
The CVS Caremark formulary change provides a real-world case study in how payer decisions shape treatment trajectories for patients with obesity. Between June and July 2025, when the formulary change was implemented, switching from AOM tirzepatide increased, while patients with an active supply of AOM semaglutide showed stable or even slightly reduced switching during the same period, consistent with its designation as the preferred option. Among patients who switched from AOM tirzepatide to a new therapy, the majority transitioned to AOM semaglutide, aligning with its status as the CVS Caremark preferred therapy.
These findings build on prior observations of GLP-1 RA switching during periods of supply constraint. Historically, shortages prompted substitution across drug classes, with patient experience shaped largely by availability. The current formulary-driven switch, in contrast, represents a payer-initiated formulary shift rather than a supply-side limitation. This distinction underscores the evolving set of non-clinical factors—pricing strategies, payer negotiations, and formulary design—that increasingly influence therapeutic choices in diabetes and obesity care. As many patients receive medication supplies greater than one month, it will be important to continue to track these trends. We expect continued additional medication switching in the coming months.
CVS Caremark manages prescription benefits for more than 25-30 million Americans, meaning this formulary change could directly affect tens of millions of people (10). Patients currently using AOM tirzepatide may need to switch to AOM semaglutide to maintain insurance coverage of the medication, while those wishing to remain on AOM tirzepatide may face higher out-of-pocket costs or coverage denials. CVS Caremark has characterized this change as an effort to lower out-of-pocket drug costs and broaden access to weight management therapies. This change also reflects potential considerations regarding tolerability, clinical outcomes, and affordability. In clinical trials, AOM tirzepatide has demonstrated greater weight loss efficacy compared with AOM semaglutide, suggesting that some patients transitioning therapies may experience different outcomes (11, 12). The change could also introduce a new administrative considerations for patients and providers as they evaluate alternatives or navigate exceptions.
This analysis has several limitations. First, dispense data are ingested by Truveta health systems often at the point of care. Therefore, these data have delays and often require a patient encounter to have occurred prior to being ingested. Our analysis represents patients who had these data available; these patients had more recent interactions, and therefore, may be less healthy than the general population receiving GLP-1s. However, we do not have a reason to believe that switching patterns would be different between these groups. This analysis only focused on switching patterns and did not look into patients who discontinued their GLP-1 RA in association with this change; therefore, we may be underestimating the extent of therapy changes. Finally, the observed changes in switching behavior occurred at the same time as the CVS Caremark formulary change; however, this analysis did not evaluate causality. We did not include an analysis of patient payor; and therefore, we cannot confirm that the CVS formulary change directly caused each medication switch. Other factors—such as supply dynamics, regional payor policies, or provider preferences—may also have contributed.
These are preliminary research findings and not peer reviewed. Data are constantly changing and updating. These findings are consistent with data accessed on August 27, 2025.
Citations
- D. J. Drucker, Efficacy and safety of GLP-1 medicines for type 2 diabetes and obesity. Diabetes Care 47, 1873–1888 (2024).
- S. Patel, S. K. Niazi, Emerging Frontiers in GLP-1 Therapeutics: A Comprehensive Evidence Base (2025). Pharmaceutics 17, 1036 (2025).
- M. M. Wong, C. Tu, Y. Li, R. L. Perlman, R. Pecoits-Filho, A. A. Lopes, I. Narita, H. Reichel, F. K. Port, N. Sukul, Anemia and iron deficiency among chronic kidney disease Stages 3–5ND patients in the Chronic Kidney Disease Outcomes and Practice Patterns Study: often unmeasured, variably treated. Clinical Kidney Journal 13, 613–624 (2020).
- A. M. Jastreboff, L. J. Aronne, N. N. Ahmad, S. Wharton, L. Connery, B. Alves, A. Kiyosue, S. Zhang, B. Liu, M. C. Bunck, A. Stefanski, Tirzepatide Once Weekly for the Treatment of Obesity. N Engl J Med 387, 205–216 (2022).
- E. D. Michos, F. Lopez‐Jimenez, M. Gulati, Role of Glucagon‐Like Peptide‐1 Receptor Agonists in Achieving Weight Loss and Improving Cardiovascular Outcomes in People With Overweight and Obesity. JAHA 12, e029282 (2023).
- S. Gratzl, B. M. G. Cartwright, P. J. Rodriguez, K. Gilbert, D. Do, N. Masters, N. L. Stucky, Monitoring Report: GLP-1 RA Prescribing Trends – June 2025 Data. medRxiv, 2025.03.06.25323524 (2025).
- A. Montero, G. Sparks, M. Presiado, L. Hamel, KFF Health Tracking Poll May 2024: The Public’s Use and Views of GLP-1 Drugs, Poll Finding (2024). https://www.kff.org/health-costs/kff-health-tracking-poll-may-2024-the-publics-use-and-views-of-glp-1-drugs/.
- F. Kansteiner, In major blow for Lilly, Novo strikes deal giving Wegovy preferred access on CVS Caremark formulary, Fierce Pharma (2025). https://www.fiercepharma.com/pharma/major-blow-lilly-novo-strikes-deal-giving-wegovy-preferred-access-cvs-caremark-formulary.
- Improving access and affordability to high-cost weight management drugs. https://www.cvshealth.com/news/pbm/improving-access-and-affordability-to-high-cost-weight-management-drugs.html.
- A. Bratulic, Lilly’s Q1 revenue surge overshadowed by CVS dumping Zepbound for Wegovy | FirstWord Pharma, FirstWord Pharma (2025). https://firstwordpharma.com/story/5955788.
- P. J. Rodriguez, B. M. G. Cartwright, S. Gratzl, R. Brar, C. Baker, T. J. Gluckman, N. L. Stucky, Semaglutide vs tirzepatide for weight loss in adults with overweight or obesity. JAMA Internal Medicine 184, 1056–1064 (2024).
- A. Singh, A. K. Singh, R. Singh, A. Misra, Comparative efficacy and safety of semaglutide 2.4 mg and tirzepatide 5–15 mg in obesity with or without type 2 diabetes: A systematic review of Phase 3 clinical trials. Diabetes & Metabolic Syndrome: Clinical Research & Reviews 19, 103212 (2025).


