- Nearly one-quarter of patients exceeded the 140 mg/dL threshold on the 1-hour glucose tolerance test. However, about 1 in 10 patients with elevated values did not receive recommended follow-up testing or a GDM diagnosis, highlighting gaps in adherence to clinical guidelines.
- Overall, 8.2% of the population in this study had gestational diabetes.
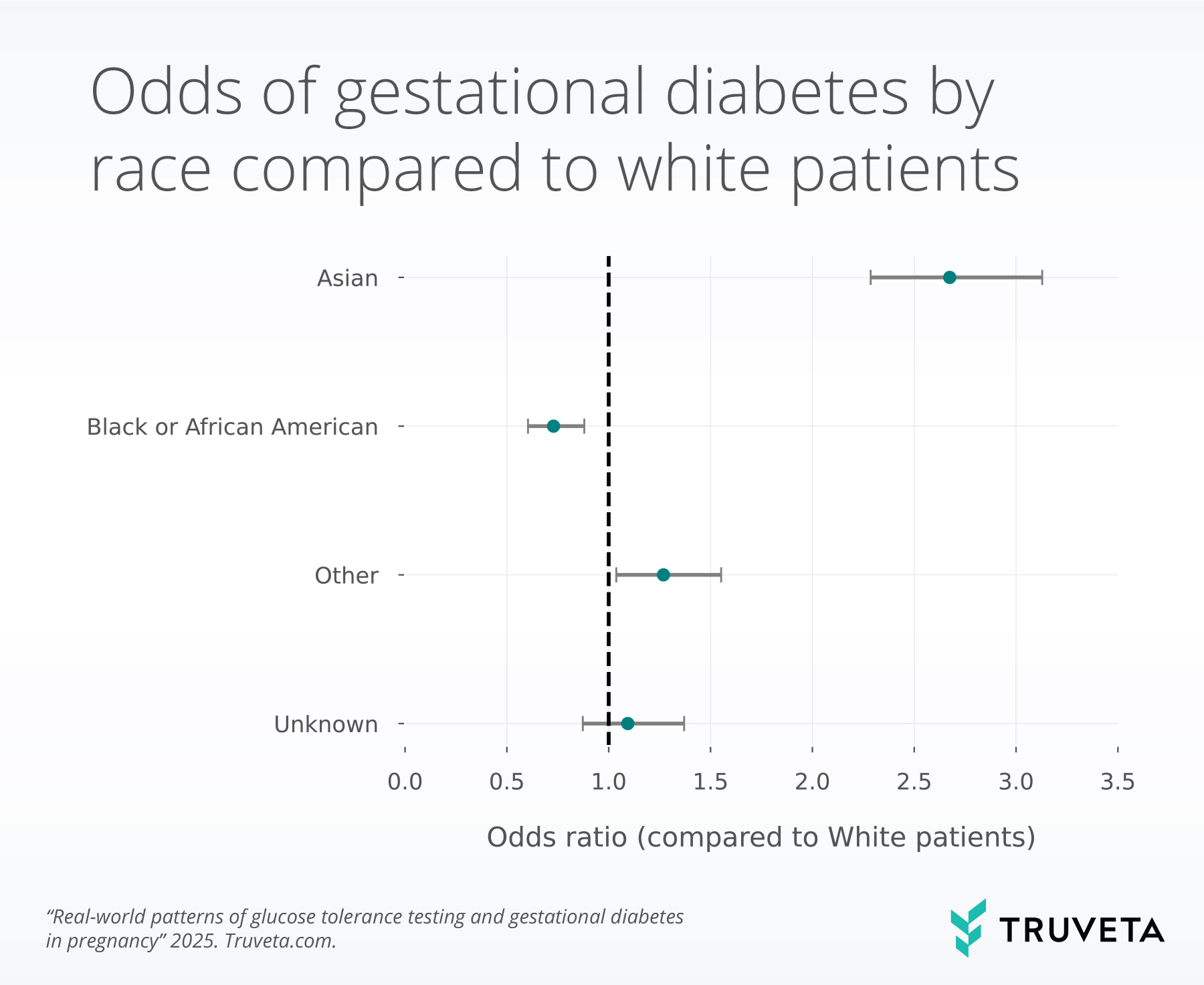
- Asian patients had significantly higher 1-hour GTT values and 2.7× higher odds of gestational diabetes compared to White patients. These differences persisted after adjusting for age, BMI, and ethnicity.
Gestational diabetes mellitus (GDM) is one of the most common complications of pregnancy, affecting an estimated 6–9% of pregnancies in the United States (1). GDM is associated with increased risk of hypertensive disorders, cesarean delivery, and large-for-gestational-age infants (macrosomia) in the short term, as well as long-term risk of type 2 diabetes for both the mother and child (1–3). Because of these consequences, early identification and appropriate management are critical.
Screening for GDM typically begins with a 1-hour glucose tolerance test (GTT) between 24 and 28 weeks of gestation (1). Women with abnormal screening results are usually referred for a 3-hour glucose tolerance test (GTT) to confirm diagnosis. However, there is variation in clinical practice regarding thresholds for abnormal values (130 mg/dL vs 140 mg/dL) (4, 5) and the 3-hour GTT (6, 7). This variability can influence who is diagnosed, how they are managed, and ultimately, maternal and neonatal outcomes.
Disparities in the risk and diagnosis of GDM by race, ethnicity, and maternal characteristics have been well documented (8, 9). For example, Asian women are more likely to be diagnosed with GDM compared to White women, even after accounting for BMI (9, 10). Understanding how glucose values and follow-up testing differ across demographic groups is essential for identifying gaps in care and ensuring equitable outcomes.
Using a subset of Truveta Data, we evaluated patterns of glucose testing and diagnosis in first pregnancies between 2018 and 2025. Our goals were to:
- Describe the distribution of values for the 1-hour and 3-hour GTTs
- Assess variation in follow-up testing after an abnormal 1-hour GTT
- Compare odds of GDM across demographic and clinical subgroups, adjusting for age and pre-pregnancy BMI
This analysis uses hourly lab values from the 1-hour and 3-hour GTTs to provide a nuanced look at variation in clinical patterns and highlight demographic groups at higher risk for GDM. You can also view this study directly in Truveta Studio.
Methods
Using a subset of Truveta Data, we included a cohort of women who had evidence of the 1-hour GTT between 2018 and August 2025 and were between the ages of 16 – 50 years during their first pregnancy. Patients were required to have a gestational age code (Z3A), which was used to estimate gestational age. Patients were also required to have linked continuous coverage of closed medical claims from 24 – 42 weeks gestation. Women were excluded if they had evidence of type 1 or 2 diabetes, a claim for a glucose monitor, nutrition counseling, or a prescription or medication fill of insulin or metformin prior to the date of their first glucose test.
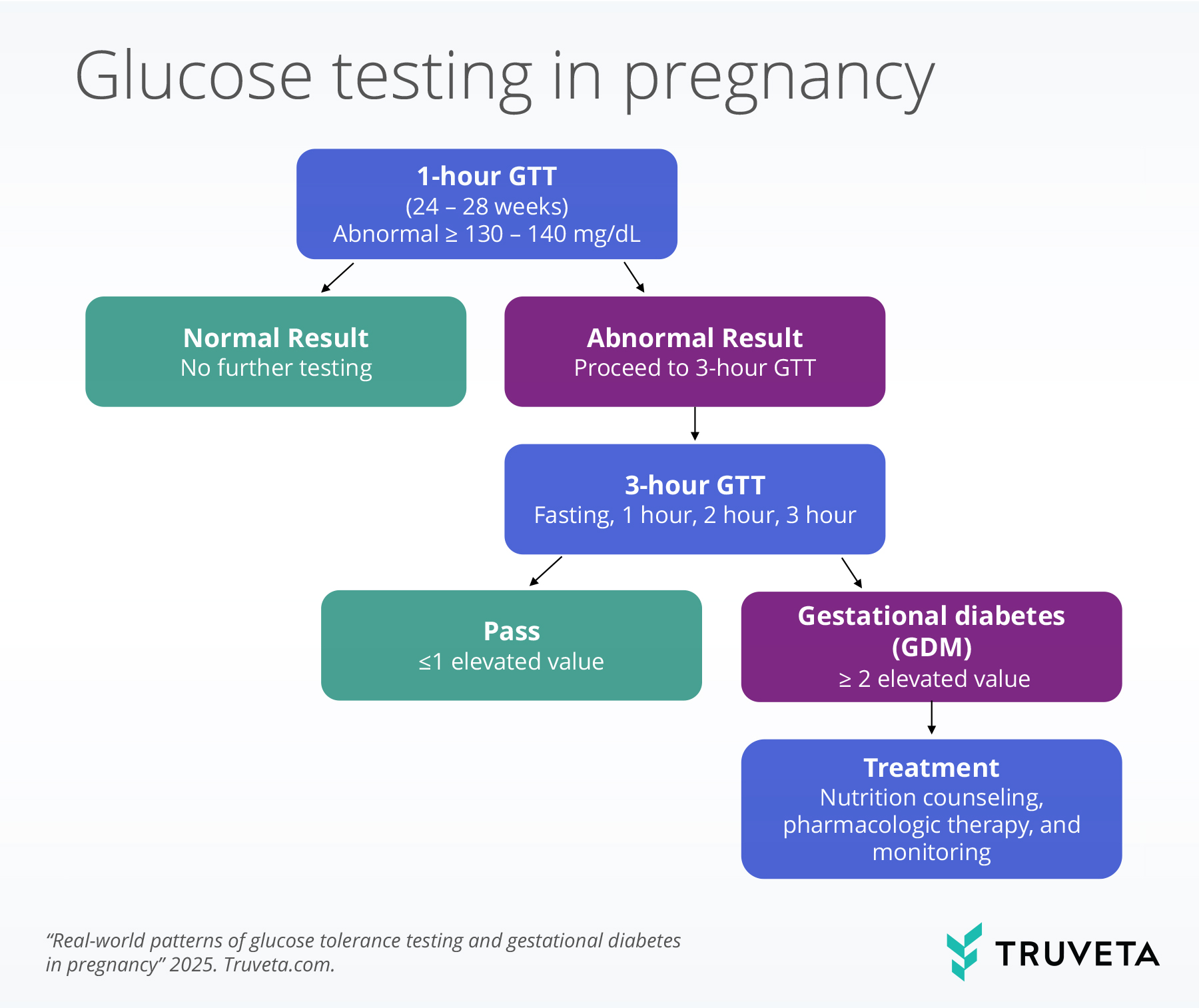
Screening for gestational diabetes typically starts with a 1-hour GTT test at 24–28 weeks; women with abnormal results are referred for a 3-hour test, where two or more elevated values confirm a diagnosis and guide next steps in management. The flow chart below illustrates the clinical pathway for glucose testing in pregnancy.
1-hour glucose tolerance test values
For both the 1-hour and 3-hour GTTs, results were standardized to mg/dL, and implausible values (<54 or >400 mg/dL) were excluded. The 1-hour GTT was required to occur between 23 and 29 weeks of gestation to allow for one week of variation on each side of the recommended gestational age at testing (24-28 weeks). A value above 140 mg/dL is generally considered abnormal, although some clinical practices use a stricter threshold of 130 mg/dL.
To assess differences in glucose levels, we fit a linear regression model adjusting for race, ethnicity, maternal age, and pre-pregnancy BMI.
3-hour glucose tolerance test values
If a patient has a high 1-hour GTT, a 3-hour GTT is typically recommended. This test includes four blood draws: a fasting draw prior to drinking the 100g glucose beverage, followed by measurements at 1, 2, and 3 hours after drinking the beverage. The Carpenter-Coustan (CC) criteria are the most commonly used thresholds (4, 11). Elevated values at each draw are: ≥95 mg/dL for fasting, ≥180 mg/dL for 1 hour, ≥155 mg/dL for 2 hours, and ≥140 mg/dL for 3 hours, although diagnostic cutoffs may vary slightly across clinical practices (4, 11). Gestational diabetes is diagnosed when two or more values meet or exceed these thresholds (4). We report the rates of follow-up GTTs, based on initial 1-hour GTT.
We classified participants into four outcome groups based on their 3-hour GTT results:
- Pass: All four values were below diagnostic thresholds.
- Pass, 1 high: Three of four values were below thresholds (i.e., one elevated value).
- Fail, hour 1 only: The hour 1 value was elevated, no subsequent values were available (hours 2 and 3 not performed), and the patient had evidence of a gestational diabetes diagnosis.
- Fail, ≥2 high: Two or more values were above diagnostic thresholds.
We show the distribution of each of the four lab values that occur as part of the 3-hour GTT, stratified by each outcome group.
We used a logistic regression model to compare the odds of having GDM, defined as failing the 3-hour GTT or having a GDM diagnosis, when controlling for race, ethnicity, age, and starting BMI.
Results
We included a cohort of 61,013 women with evidence of a 1-hour GTT and continuous claims enrollment. Of these, 49.4% had commercial or private insurance, 32.4% were enrolled in Medicare or Medicaid, and 18.2% had other coverage, which may have reflected multiple coverage types or unknown coverage.
1-hour glucose tolerance test
Most patients passed with lab values below 130mg/dL (68.4%). About a quarter of patients had values above the 140mg/dL threshold (21.9%), and 9.7% of patients had values between 130 and 140 (a range in which some clinicians would recommend additional testing). Higher 1-hour GTT results were observed among Asian patients compared to White patients (coefficient = 12.4, p < 0.001), when controlling for ethnicity, age, and BMI. Increased age (coefficient = 0.7, p< 0.001), BMI (coefficient = 0.8, p<0.001), and Hispanic or Latino ethnicity (coefficient = 3.7, p <0.001) also were associated with higher 1-hour GTT values. Black or African American patients had lower 1-hour GTT values compared to White patients (coefficient = -5.9, p <0.001).
Of the patients with values between 130-140mg/dL in the 1-hour GTT, 16.1% either received a GDM diagnosis or went on to have the 3-hour GTT, while 88.2% of patients with values higher than 140mg/dL either received a GDM diagnosis or had a 3-hour GTT. These findings highlight that approximately 1 in 10 patients with elevated 1-hour GTT values did not undergo guideline-directed follow-up testing or receive a GDM diagnosis. Further, there is variation in the lower threshold used to recommend additional testing. Additionally, patients with higher 1-hour GTT tests had higher rates of GDM diagnosis, without the follow-up 3-hour GTT: 15.7% of patients who had 1-hour GTT test values between 180 -190mg/dL received the diagnosis without additional testing compared to only 2.9% of patients with values between 140-150mg/dL. Similar rates of GDM diagnosis and follow-up testing were seen across racial and ethnic groups.
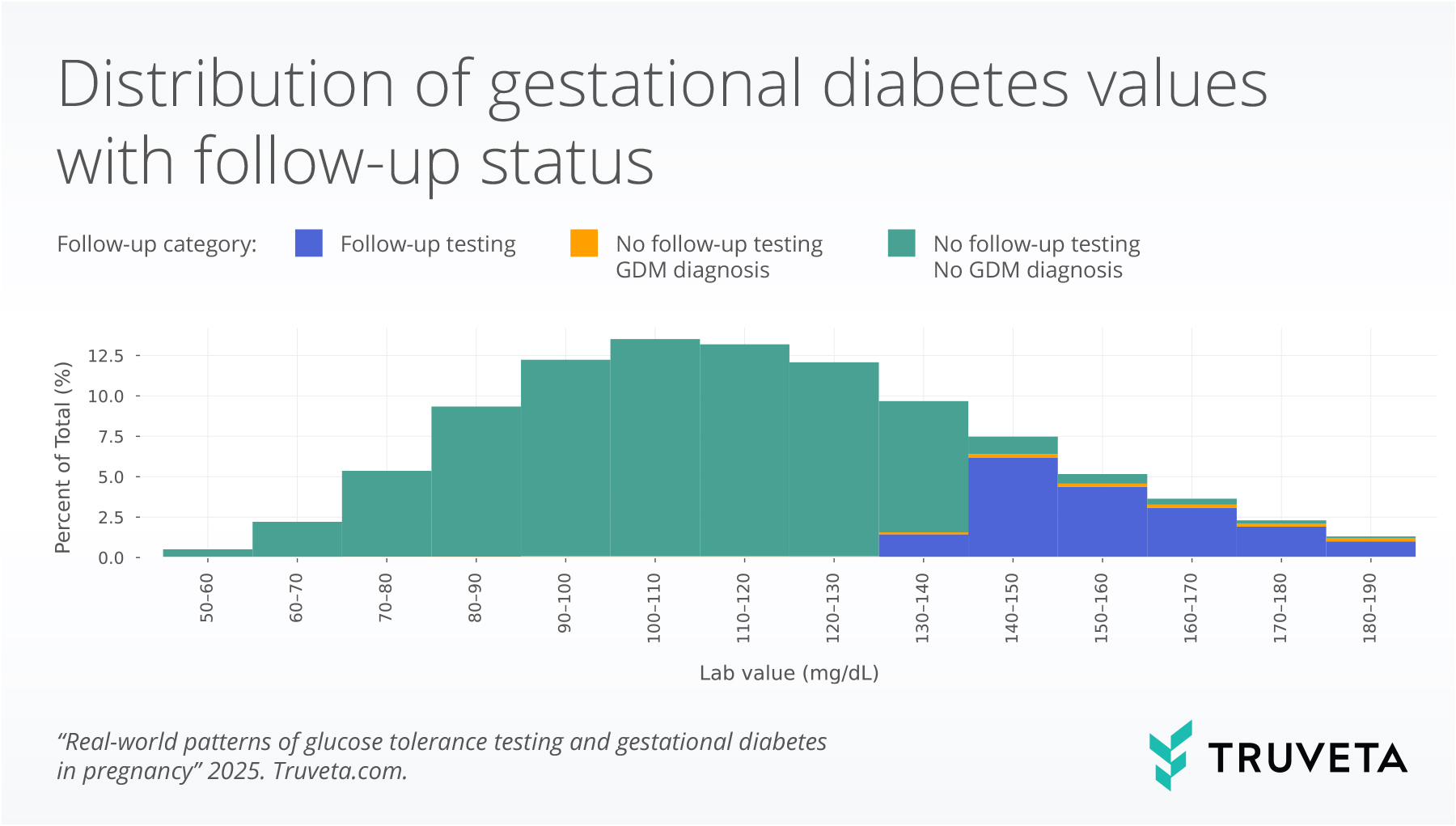
3-hour glucose tolerance test
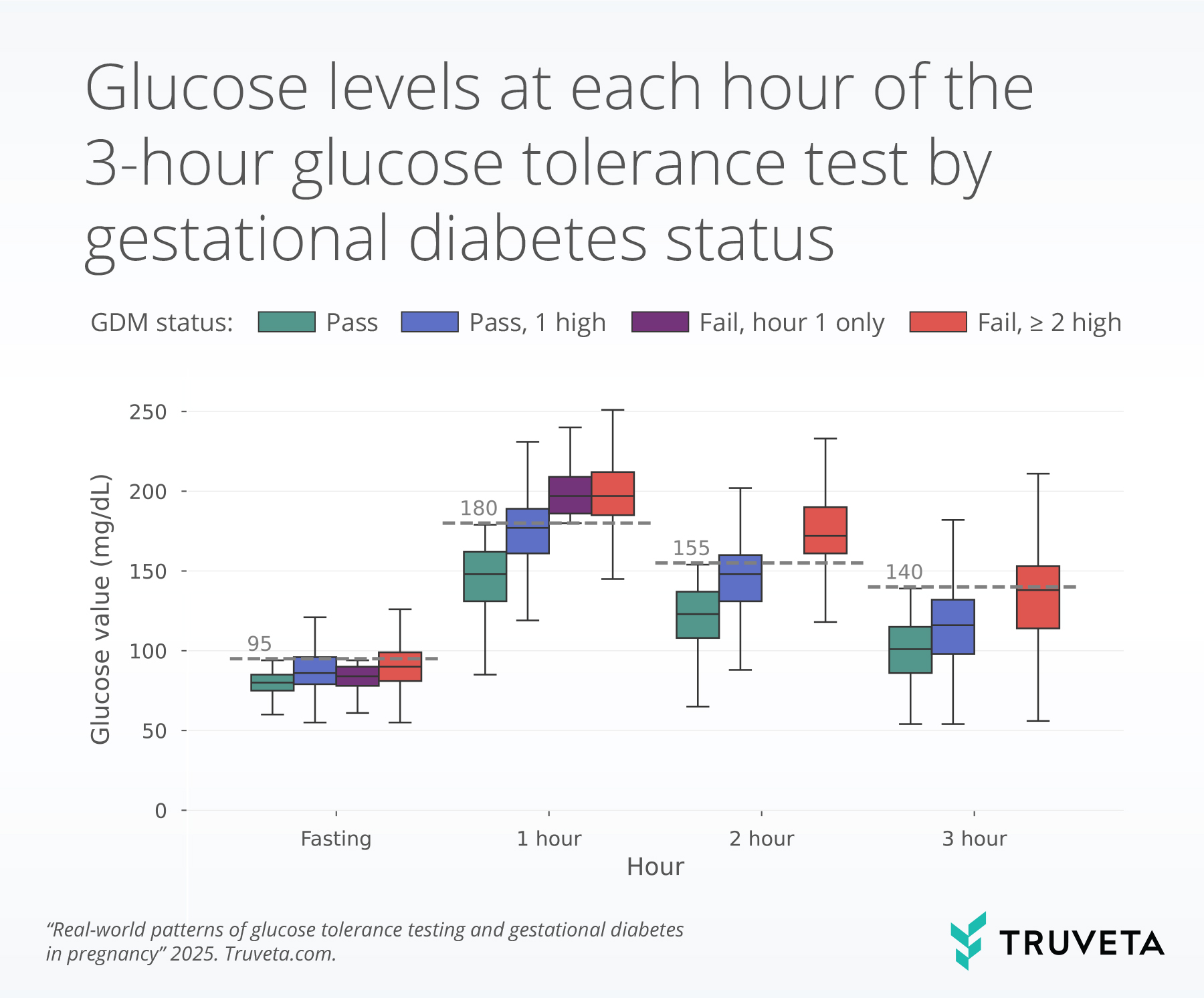
Of the patients who received the 1-hour GTT, 11,500 went on to have a 3-hour GTT. Most patients passed (78.3%). About a quarter (22.0%) of patients tested passed but with one elevated glucose value. A small group of patients (1.7%) had an elevated value during the first hour of the 3-hour GTT, did not complete the full 3-hour GTT, and received a GDM diagnosis. This group is referred to as ‘Fail, hour 1 only’ in the boxplot below.
The boxplot below shows 3-hour GTT values for each hour of the test, by testing group. The line inside each box represents the median value (or the 50th percentile). The bottom and top of the box mark the 25th and 75th percentile. Together the vertical space inside the box represents the middle half of all women. This is known as the interquartile range. The whiskers, or thin lines extending from the box, show typical lower and higher values, reaching up to 1.5 times the height of the box. The grey dashed line for each hour indicates the threshold for elevated values (i.e., 95 mg/dL for fasting).
Odds of gestational diabetes
Overall, 8.2% of the population in this study had GDM; however, there were differences by race. People of Asian race had a higher prevalence of GDM (15.3%), compared to people of White (7.5%) or Black / African American race (6.6%).
We used logistic regression to test for group differences. In this context, an odds ratio (OR) > 1 indicates higher odds of gestational diabetes mellitus (GDM) compared with the reference group, while an OR < 1 indicates lower odds. When the confidence interval (grey bars in the image below) overlaps the dashed line at 1, the association is not statistically significant.
We found that race was significantly associated with the odds of GDM, when controlling for ethnicity, age, and starting BMI. Compared to White patients, Asian patients had significantly higher odds of GDM (odds ratio [OR] = 2.7, p < 0.001), and Black or African American patients had lower odds of GDM (OR = .7, p=0.001). We also observed small increases associated with higher pre-pregnancy BMI (OR = 1.06 p<0.001) and age (1.06, p = 0.01) and Hispanic ethnicity (OR = 1.4, p<0.001).
Discussion
In this large cohort of more than 61,000 women, most patients had 1-hour GTT values within the normal range. However, nearly one-quarter exceeded the widely used 140 mg/dL threshold, warranting follow-up testing. Among those with abnormal results, not all underwent a 3-hour confirmatory test. Notably, about 1 in 10 women with elevated values did not receive follow-up testing nor a GDM diagnosis, which falls short of standard care. In some cases, women with particularly high 1-hour values were diagnosed directly with gestational diabetes mellitus (GDM), while others with borderline values between 130–140 mg/dL proceeded to additional testing. These variations in screening thresholds and referral practices across the United States may contribute to inconsistencies in how GDM is diagnosed and managed.
Our analysis also revealed important differences across demographic subgroups. Asian patients had significantly higher 1-hour GTT values and greater odds of GDM compared to White patients, even after adjustment for age, pre-pregnancy BMI, and ethnicity. In contrast, Black or African American patients had lower 1-hour values. These findings align with prior studies showing that Asian women have a higher risk of GDM despite lower average BMI (9, 10, 12). Such patterns raise important questions about whether current diagnostic thresholds or treatment recommendations are appropriately calibrated across racial and ethnic groups (12).
Our findings underscore the importance of standardized, evidence-based screening protocols for GDM, as well as the need to better understand disparities in testing and diagnosis across patient groups. Variation in clinical practice can lead to underdiagnosis in some populations and unnecessary interventions in others. Future research should examine whether differences in screening thresholds, screening timing, and follow-up contribute to disparities in interventions, such as nutrition counseling, maternal and neonatal outcomes, and whether alternative approaches—such as risk-based screening or modified cutoffs for high-risk groups—could improve equity and effectiveness in GDM care.
There are a few limitations associated with this study. First, to be included in the study women were required to have continuous linked medical claims coverage. If a women changed coverage between 24 – 42 weeks gestation and either the first or second coverage was not part of the linked data, she would not be included in this study. Additionally, women who are uninsured are not captured in these data. We also required the 1-hour GTT to occur between 23 – 29 weeks gestation. We allowed a one-week buffer around the recommended window of 24 – 28 weeks (4); however, if a woman deviated from the standard recommended clinical care guidelines, she would not be included. Finally, if the 3-hour GTT happened outside a Truveta member health system, it would not be captured in this analysis; however, it is relatively unlikely to receive the follow up 3-hour GTT at a different facility than the 1-hour GTT.
Despite these limitations, this study has notable strengths, including its large population size and direct usage of individual lab values, not just a pass / fail test result. By capturing real-world variation in screening thresholds, follow-up testing, and diagnostic practices, our analysis provides unique insight into how current approaches to GDM screening may contribute to inequities in care. Our findings highlight both variability in care as well as how clinical practice may intersect with patients’ demographic differences, offering an important foundation for future work to refine and standardize GDM screening protocols.
In a follow-on analysis, we explore the impact of gestational diabetes treatments—such as nutrition counseling, insulin, and metformin—on post-glucose tolerance testing weight gain among pregnant patients.
These are preliminary research findings and not peer reviewed. Data are constantly changing and updating. These findings are consistent with data accessed on September 30, 2025.
Citations
- Centers for Disease Control and Prevention, Gestational Diabetes (2024). https://www.cdc.gov/diabetes/about/gestational-diabetes.html.
- The HAPO Study Cooperative Research Group, Hyperglycemia and Adverse Pregnancy Outcomes. N Engl J Med 358, 1991–2002 (2008).
- L. Bellamy, J.-P. Casas, A. D. Hingorani, D. Williams, Type 2 diabetes mellitus after gestational diabetes: a systematic review and meta-analysis. The Lancet 373, 1773–1779 (2009).
- ACOG Practice Bulletin No. 190: Gestational Diabetes Mellitus. Obstetrics & Gynecology 131, e49–e64 (2018).
- J. S. Will, H. Crellin, Gestational diabetes mellitus: update on screening, diagnosis, and management. American Family Physician 108, 249–258 (2003).
- C. Caissutti, V. Berghella, Scientific Evidence for Different Options for GDM Screening and Management: Controversies and Review of the Literature. BioMed Research International 2017, 1–12 (2017).
- L. Li-zhen, X. Yun, Z. Xiao-Dong, H. Shu-bin, W. Zi-lian, D. Adrian Sandra, L. Bin, Evaluation of guidelines on the screening and diagnosis of gestational diabetes mellitus: systematic review. BMJ Open 9, e023014 (2019).
- A. Ferrara, Increasing Prevalence of Gestational Diabetes Mellitus. Diabetes Care 30, S141–S146 (2007).
- M. Hedderson, S. Ehrlich, S. Sridhar, J. Darbinian, S. Moore, A. Ferrara, Racial/Ethnic Disparities in the Prevalence of Gestational Diabetes Mellitus by BMI. Diabetes Care 35, 1492–1498 (2012).
- S. Y. Chu, K. Abe, L. R. Hall, S. Y. Kim, T. Njoroge, C. Qin, Gestational diabetes mellitus: All Asians are not alike. Preventive Medicine 49, 265–268 (2009).
- M. W. Carpenter, D. R. Coustan, Criteria for screening tests for gestational diabetes. American Journal of Obstetrics and Gynecology 144, 768–773 (1982).
- J. K. Silva, J. K. Kaholokula, R. Ratner, M. Mau, Ethnic Differences in Perinatal Outcome of Gestational Diabetes Mellitus. Diabetes Care 29, 2058–2063 (2006).

