In May 2025, the FDA cleared the first blood test to aid in diagnosing Alzheimer’s disease, making early detection more accessible than ever. The Lumipulse test measures biomarkers in the blood associated with Alzheimer’s pathology, offering a simpler alternative to expensive brain scans or invasive lumbar punctures.
Next generation blood tests are a promising leap forward, but also raise new questions:
- How will blood tests like Lumipulse perform in real-world practice, across diverse patients and health systems?
- Will it reach the patients who need it most, or will disparities emerge in access?
- How do results align with real-world cognitive scores, imaging findings, and disease progression?
Answering these questions — and turning diagnostics innovation into better patient outcomes — requires robust, linked real-world data that can track adoption and outcomes in real time.
Tracking adoption and disparities with real-world data
With the most representative, complete, and timely patient journey data, Truveta empowers researchers and regulators to evaluate new diagnostics like Lumipulse at scale and without the long lag times of other traditional datasets.
With EHR data covering more than 569,000 patients with Alzheimer’s disease, Truveta Data includes:
- Cognitive scores (MMSE, MoCA, SLUMS) — essential for tracking real-world disease severity and validating diagnostic performance.
- Linked imaging and blood-based biomarker data — including hundreds of thousands of brain imaging studies for patients with dementia (such as PET and MRI), along with lab results for p-tau181, p-tau217, Aβ40, Aβ42, and Aβ42/Aβ40 ratios.
- Linked claims and SDOH data — enabling analysis of disparities in access and outcomes.
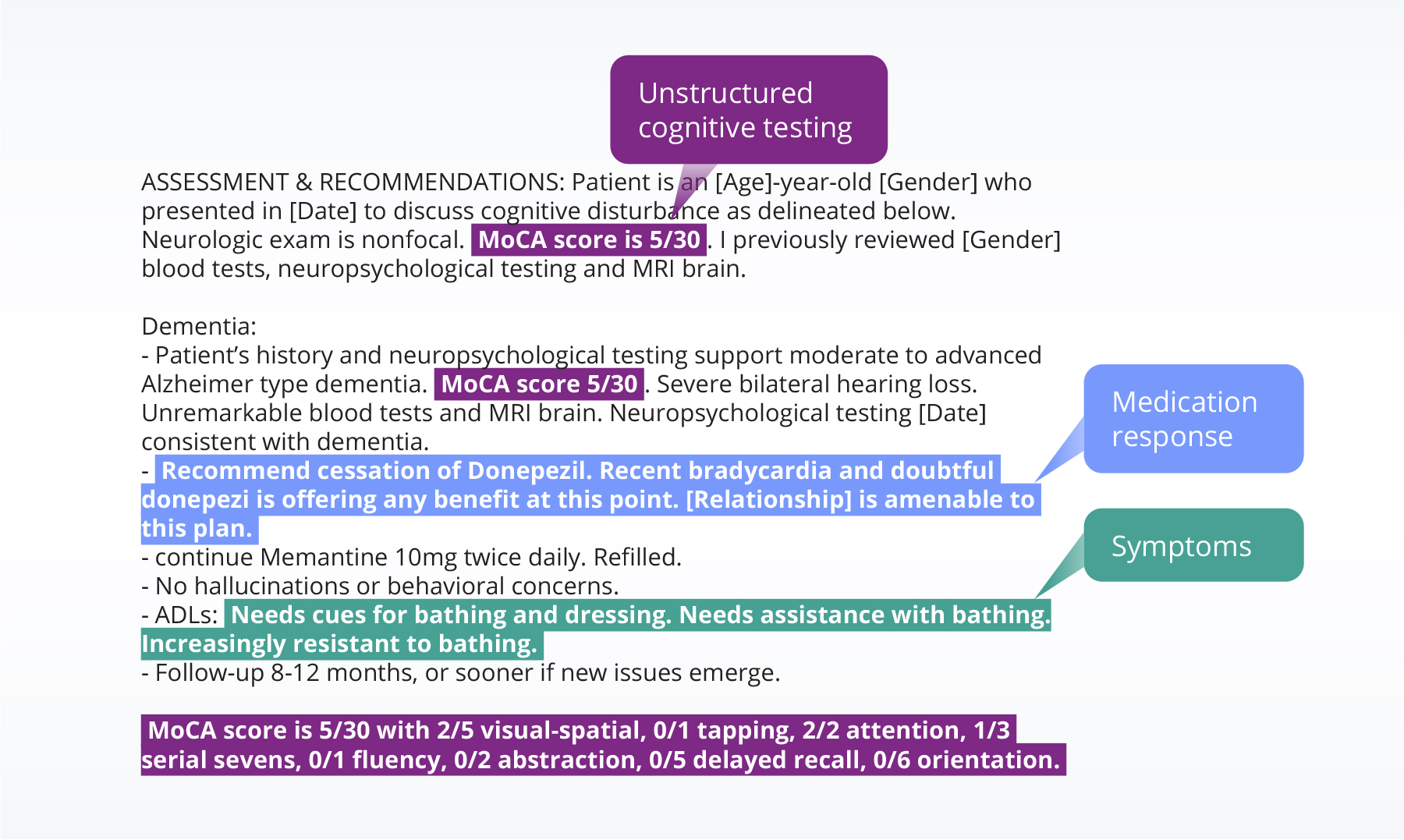
- Clinical notes integrated with EHR data— capturing nuanced context like APOE status, symptoms, living situation, and family history.
Together, these data provide an unprecedented ability to monitor the adoption and impact of Alzheimer’s diagnostics and treatments across diverse populations.
Real-time insights in action: Tracking the uptake of lecanemab
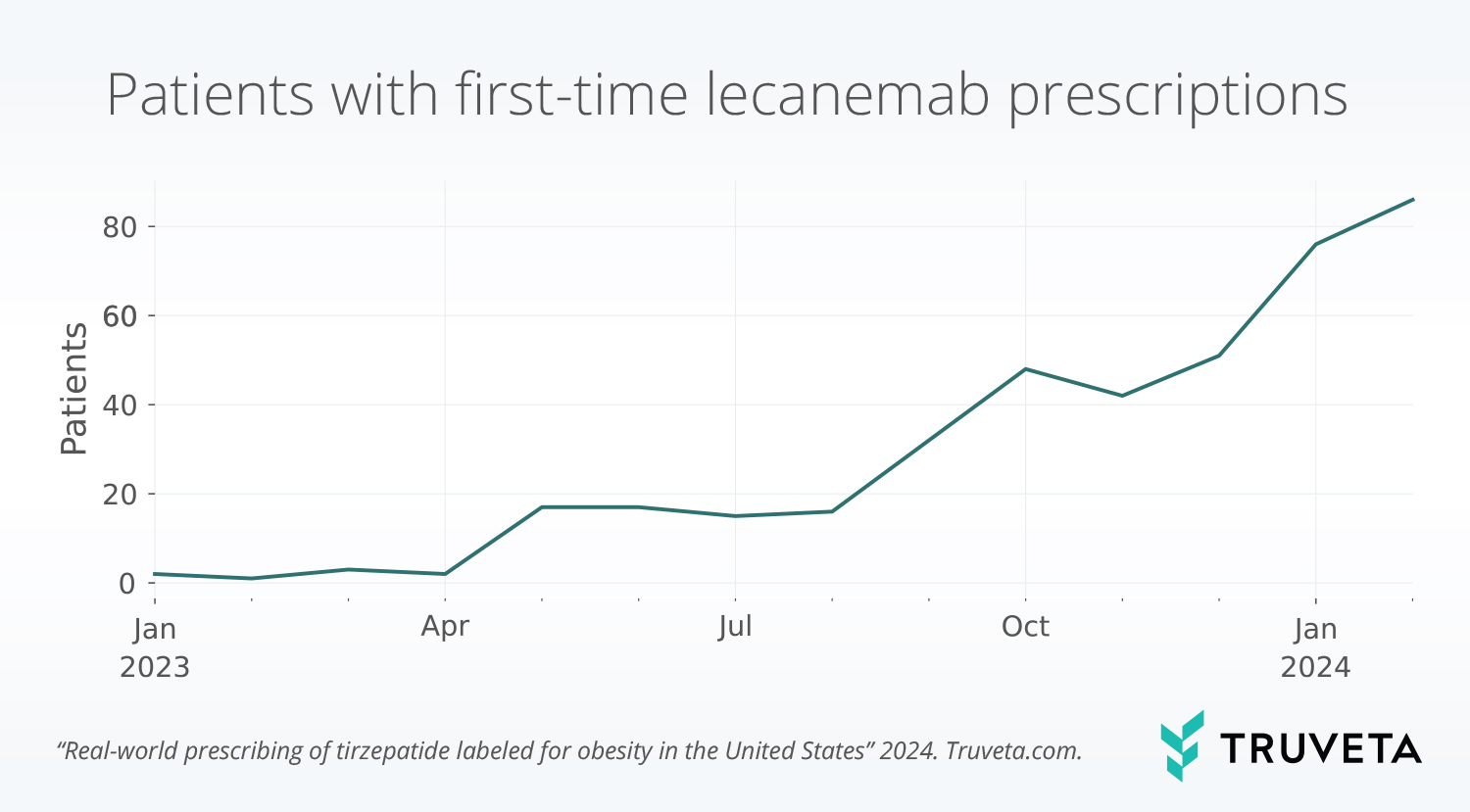
To illustrate the power of real-world data, Truveta Research analyzed the real-world uptake of lecanemab— a monoclonal antibody treatment approved for Alzheimer’s in 2023. The results highlight both momentum and inequity:
- Uptake accelerated after FDA approval, with prescriptions peaking in early 2024.
- Patients prescribed lecanemab were more likely to be white, younger than 74, with higher income and housing stability.
- These patterns underscore how real-world barriers can limit access to new therapies — and could also shape how new diagnostic tests are used in practice.
Use cases for pharma
Truveta Data supports a range of strategic priorities across pharma teams working in Alzheimer’s disease:
- Monitor post-market test and treatment adoption across health systems in real time
- Support label expansion and HEOR analysis with linked imaging, biomarkers, and cognitive measures
- Detect access disparities to anticipate payer and regulatory questions
- Stratify patients by APOE genotype, amyloid/tau ratios, cognitive status, or social determinants
- Build synthetic control arms using real-world data matched to trial-eligible populations
- Accelerate trial enrichment by identifying eligible patients using structured and unstructured EHR data
Looking ahead
The FDA’s approval of Lumipulse signals a turning point: simple blood tests will soon be a routine part of Alzheimer’s care. To realize their full potential, providers, researchers, and policymakers need visibility into who is being tested, who is benefiting, and where care gaps remain.
That’s the role of real-world data.
By combining clinical, imaging, biomarker, claims, and SDOH data — refreshed daily and linked at scale — Truveta offers a unique platform to measure and improve the real-world impact of diagnostic innovations.
See what’s possible with Truveta
Interested in how your team can use Truveta Data for Alzheimer’s research, label expansion, or biomarker validation?
Request a personalized demo to explore how Truveta can help accelerate your next study.
