Multiple sclerosis (MS) affects approximately 1 million people in the United States, with women representing nearly 75% of patients. Over the past decade, consensus guidelines from leading MS experts have increasingly emphasized the importance of early intervention with high-efficacy disease-modifying therapies (DMTs) to slow disability progression and improve long-term outcomes.
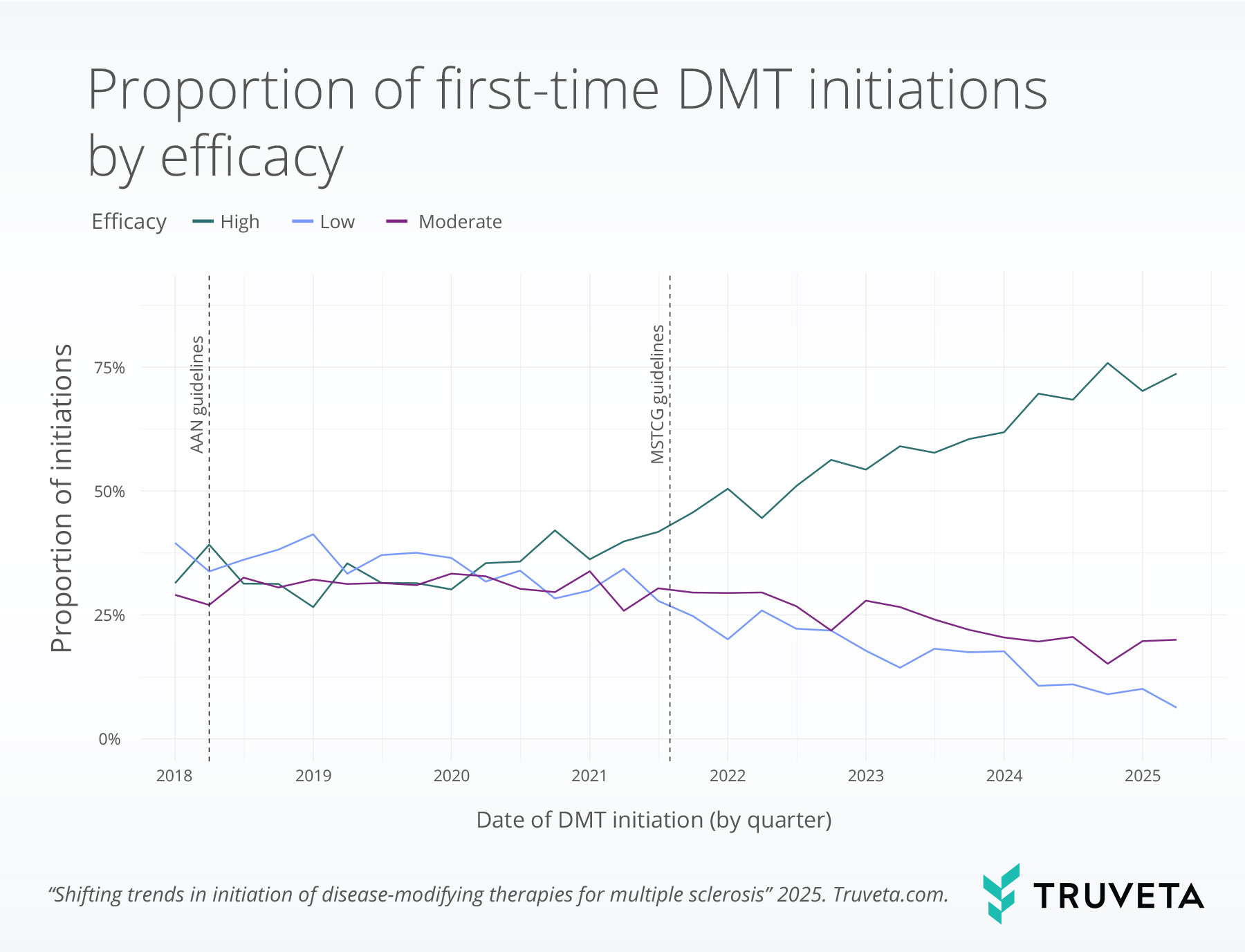
Now, new findings from Truveta Research examining DMT prescribing patterns provide real-world confirmation of this shift. Between January 2018 and June 2025, the proportion of patients initiating treatment with a high-efficacy DMT more than doubled—capturing changes in practice that many other data sources take years to reveal. Because Truveta Data is updated daily, researchers can observe market shifts and treatment trends as they are happening, rather than waiting years for lagging reports.
By showing how treatment decisions are evolving in practice, Truveta Data offers timely and clinically deep insights into whether patients are receiving therapies aligned with the latest scientific consensus and where important care gaps remain.
Understanding treatment patterns with real-world data
The MS treatment landscape is complex. Patients may cycle between therapies due to side effects, lack of efficacy, or insurance barriers. Some discontinue therapy altogether, increasing the risk of relapse and disability progression. These patterns are especially pronounced in relapsing-remitting MS (RRMS), where frequent therapy adjustments are common. Capturing the full scope of these dynamics requires more than claims data alone, it requires the integration of clinical, imaging, and patient-reported information.
Truveta Data tracks the full patient journey, including:
- Early symptoms and diagnosis delays
- MRI reports documenting new lesions
- Expanded Disability Status Scale (EDSS) scores
- Relapse timing and flare management (e.g., steroid bursts, urgent neurology visits, hospitalizations)
- Treatment initiation, switches, adherence patterns, and discontinuations
These MS-specific endpoints are essential for post-market research, health economics and outcomes research (HEOR), and for generating evidence aligned with regulatory expectations. They allow researchers to quantify not just what treatment was given, but why it changed and what outcomes followed.
Clinical notes: Unlocking the “why” in MS care
Structured data often miss the details that shape clinical decision-making. 95% of data generated by healthcare organizations goes unused. The Truveta Language Model (TLM) extracts data from notes at scale, empowering researchers with data from more than 7 billion clinical notes . This enables researchers to:
- Identify relapse indicators mentioned in provider documentation
- Analyze MRI findings
- Capture functional decline or cognitive symptoms not coded elsewhere
- Understand why therapies were stopped (e.g., side effects, safety events, pregnancy)
- Link these insights to structured data such as medications, labs, and imaging

By combining structured and unstructured data, researchers gain a more precise and contextualized view of MS care—critical for designing trials, validating endpoints, and assessing real-world effectiveness.
The most representative, complete, and timely EHR data
Truveta provides daily updated EHR data for more than 120 million patients, including clinical notes and images, linked with closed claims. This breadth and depth, sourced directly from leading US health systems, ensures consistent data quality and full traceability to the clinical source.
For MS, this means access to the full patient journey from early symptoms and MRI reports, to relapse timing and functional decline. Researchers can study treatment history (e.g., anti-CD20s, S1Ps), adherence patterns, steroid use for flare management, and evidence of relapse activity. Together, these data support robust analyses for HEOR modeling, safety surveillance, post-market commitments, and trial simulation in RRMS and progressive forms of the disease.
Looking ahead
MS care has come a long way from simply managing symptoms. Advances in DMTs transformed treatment for relapsing disease, and now the field is moving toward the pursuit of prevention and repair.
As these therapies advance, life sciences teams need real-world evidence that captures not only prescribing trends but also patient outcomes, relapse patterns, and adherence. Truveta Data enables researchers to track these outcomes at scale to evaluate new therapies and model long-term impact.
Explore more
Contact us for a custom feasibility to see how Truveta can support your MS research strategy.
