Major depressive disorder (MDD) is one of the most urgent and costly public health challenges today. More than 18% of US adults live with depression, and rates are even higher among younger adults. Despite advances in pharmacology, psychotherapy, and neuromodulation, nearly a third of patients experience treatment-resistant depression (TRD), and most available therapies take weeks before providing meaningful benefit.
In this context, new real-world evidence is essential. With representative EHR data from 1 in 3 Americans, linked closed claims, and standardized clinical measures like PHQ-9 scores, Truveta offers a holistic view into how depression is diagnosed, managed, and treated across the US, and where gaps remain. By analyzing more than 10 million patient journeys with major depressive disorder (MDD), Truveta Data can provide the evidence pharma and device innovators need—from treatment effectiveness and patient segmentation to the health economics that influence access.

What real-world evidence makes possible in MDD
Measuring effectiveness beyond the trial setting
Clinical trials establish efficacy, but real-world care is more complex. Truveta Data allows researchers to follow patients over time, linking PHQ-9 symptom trajectories with treatment adherence, discontinuation, and relapse. These insights can guide label expansions, post-market surveillance, and health economics models for antidepressants, esketamine, and emerging therapies.
Identifying patients at risk of treatment resistance
Major depressive disorder presents differently across patient symptom patterns, underlying biology, and treatment response. By combining symptom scores with comorbidities and the largest collection of clinical notes integrated with EHR data, researchers can identify subgroups at higher risk of treatment-resistant depression (TRD). This supports precision psychiatry strategies, helping innovators target costly interventions such as neuromodulation therapies to the patients most likely to benefit.
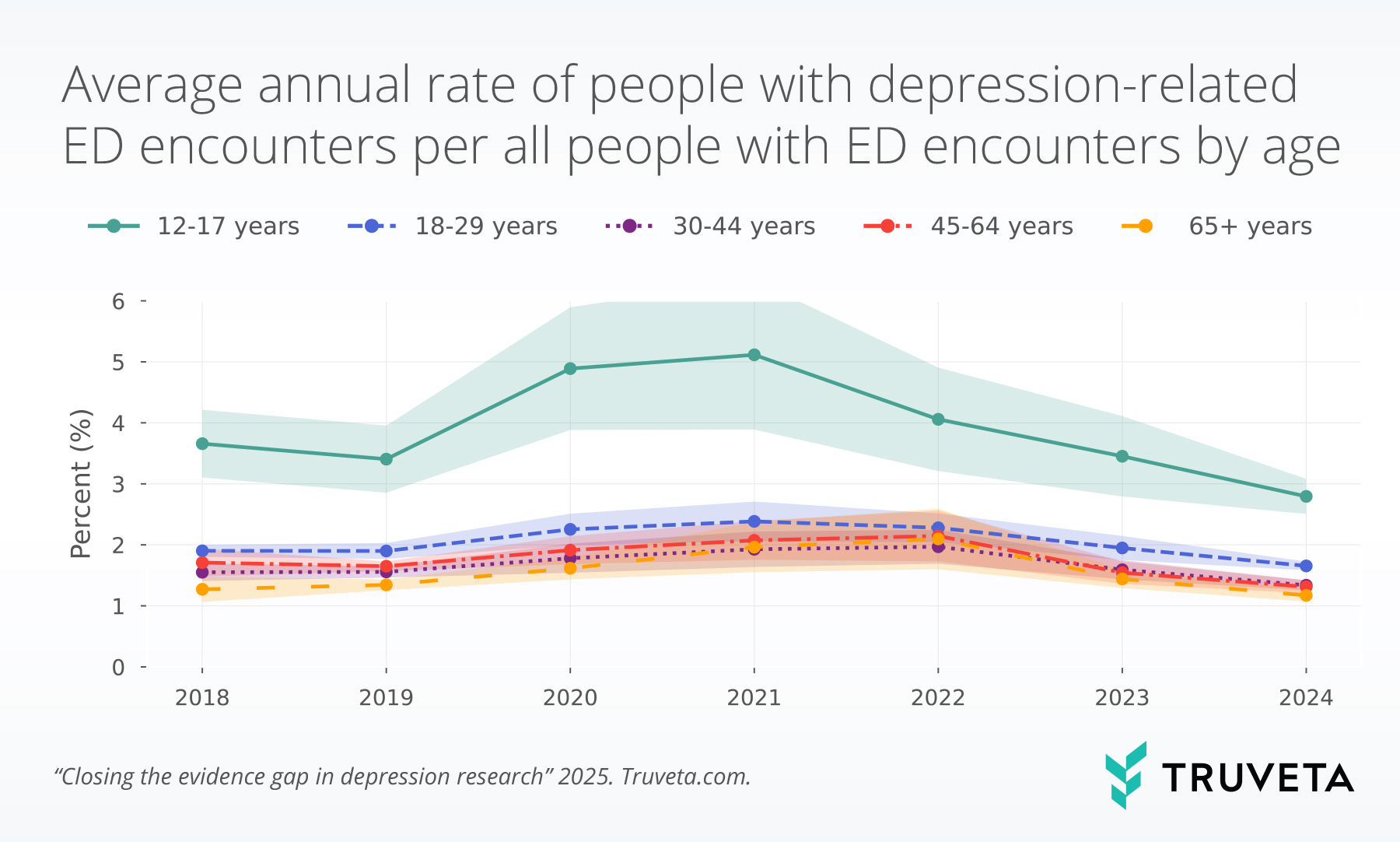
Truveta’s broader mental health analyses also highlight where depression manifests most acutely. In a recent study, Truveta Research found that adolescents (ages 12–17) had nearly twice the rate of depression-related emergency department visits compared with young adults. These findings underscore both the urgency of earlier intervention and the importance of identifying high-risk groups in real-world care.
Connecting outcomes to cost and access
Recent estimates place the annual economic burden of major depressive disorder at more than $300 billion in the United States. With nearly half (42%) of Truveta’s MDD cohort linked to closed claims, researchers can quantify how symptom improvements translate into reduced hospitalizations, readmissions, and overall cost of care. These analyses inform payer negotiations and value-based contracting, critical to broadening patient access.
Supporting digital and device innovation
As depression therapy expands beyond pills into neuromodulation and digital tools, Truveta’s unique device identifier–level data linked with symptom data open new research possibilities. Companies developing transcranial magnetic stimulation (TMS), vagus nerve stimulation (VNS), deep brain stimulation (DBS), and other implanted neurostimulators are actively pursuing indications for patients with MDD and TRD. With PHQ-9 capture and device identifier granularity, Truveta can help:
- Identify which patients are best suited for stimulation therapies
- Track symptom trajectories before and after treatment
- Support post-market studies or label expansions using real-world evidence
Why it matters
Innovation in depression care is moving fast. Regulators are increasingly open to real-world evidence and novel outcomes in mental health submissions, while payers demand proof of value in practice. Meanwhile, across therapeutic areas like oncology, cardiology, and diabetes, depression remains a critical comorbidity shaping outcomes.
With daily refreshed, representative data at scale, Truveta helps innovators answer the most pressing questions in depression research that clinical trials alone cannot resolve.
Answers can’t wait. See how Truveta Data can accelerate your next study in MDD. Contact us for a custom feasibility.
