Medical device manufacturers and regulators face a persistent challenge: obtaining a complete, clinically rich view of how devices are used, how safe they are, and how they affect patient outcomes. Historically, researchers have been limited to claims, chargemaster, and registries, which lack device-level detail, clinical context, and the timeliness needed to study safety, performance, and value in real-world settings.
With today’s expansion of Truveta Data, that compromise is no longer necessary. Truveta now offers device-level precision by integrating unique device identifier (UDI) data with minute-level admission–discharge–transfer (ADT) and chargemaster data to provide the most comprehensive view of device performance available in real-world clinical settings.
Why device data has been so limited
Device companies need evidence that is both regulatory-grade and reflective of the real-world patient journey. Traditional billing-focused datasets cannot show how and when devices are used within clinical workflows, while EHR-only data may omit important cost and billing insights.
By uniting these critical elements into a single, research-ready dataset, Truveta delivers unmatched precision, enabling more efficient regulatory submissions, faster detection of safety signals, and stronger evidence for health economics and outcomes research (HEOR).
What’s new in Truveta Data
Truveta Data now includes even more detailed data specific to devices and patient encounters to enable researchers to explore broader clinical and administrative questions:
Regulatory-grade UDI integration
More than 300,000 UDIs representing 27,000 brands are normalized through the Truveta Language Model (TLM), capturing brand, model, lot, and expiration date with full procedural context, enabling device manufacturers to study performance across product versions.
Minute-level ADT precision
Admission–discharge–transfer data provide exact timestamps for device use during surgical events, ICU transfers, recovery, and discharge—enabling detailed workflow and safety analyses.
Procedure log integration
Timestamped surgical logs provide unprecedented insight into surgical workflows and device use in context, offering detail that billing data cannot capture to inform best practices and improve standards of care.
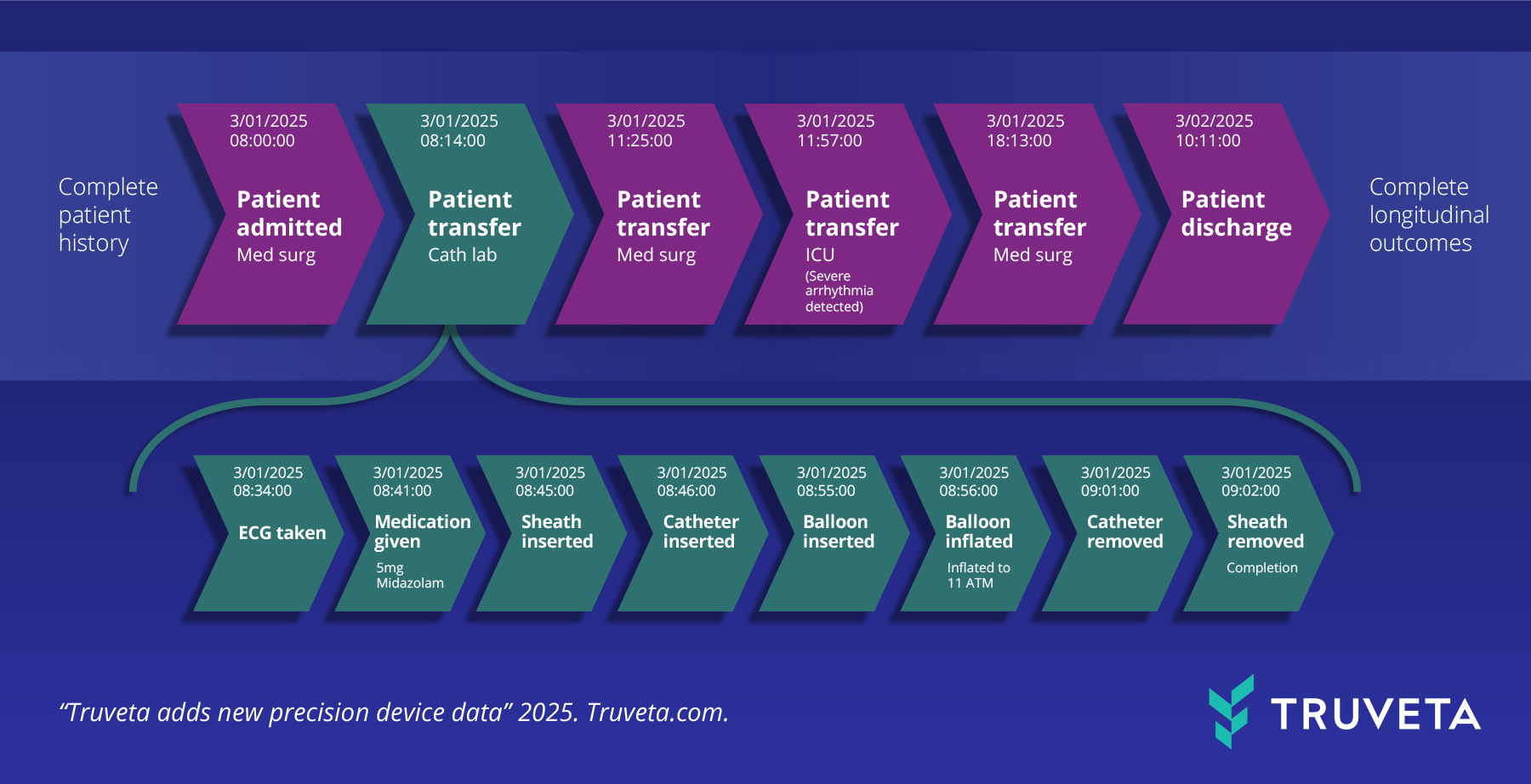
ADT and procedure log data enable the study of pre-procedural events, interoperative techniques, recovery trajectories, and outcomes.
Chargemaster data with clinical linkage
Every billed service, supply, and device is directly tied to the encounter with deep clinical context, creating a powerful foundation for HEOR and cost-effectiveness studies.
Integrated multi-modal data
Structured EHR, clinical notes, imaging, surgical logs, claims, and mortality records create the most complete patient view available. Today, more than 5 million patients in Truveta Data have both device records and associated imaging enabling device manufacturers to analyze device placement, study disease severity, and train multi-modal AI.

Redacted operative note illustrating how clinical documentation captures provider judgment and procedural nuance.
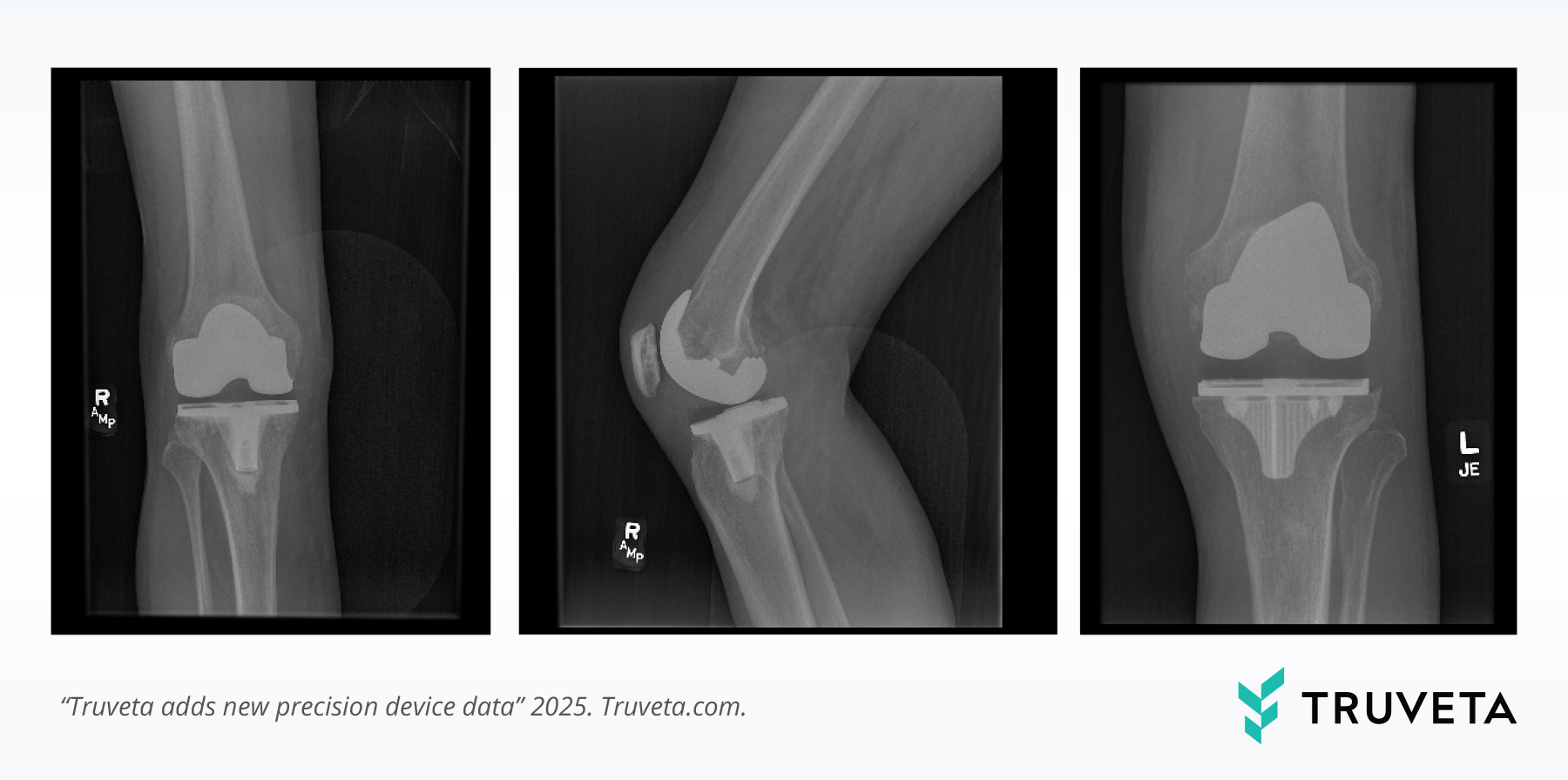
X-rays confirming prosthetic knee placement, which can be linked with device identifiers and longitudinal outcomes for comprehensive research.
The impact for medical device research
These expanded data unlock new possibilities for device research and regulation. For example, in cardiovascular care, researchers can now study the exact sequence and timing of mechanical circulatory support devices during high-risk procedures. This includes when specific device versions are deployed, how long patients are supported, and the clinical and economic outcomes that result.
These insights enable manufacturers, clinicians, and regulators to:
- Build stronger, regulatory-grade evidence with device- and minute-level precision
- Detect and act on safety signals earlier with real-time data insights
- Advance innovation that informs clinical guidelines and standards of care across diverse patient populations
Learn more
Truveta Data provides daily updated, de-identified EHR data linked with claims, mortality, and social drivers of health. With the addition of device-specific and minute-level detail, researchers now have access to the most complete and precise dataset available for medical device research.
Explore more here: Truveta Data.


