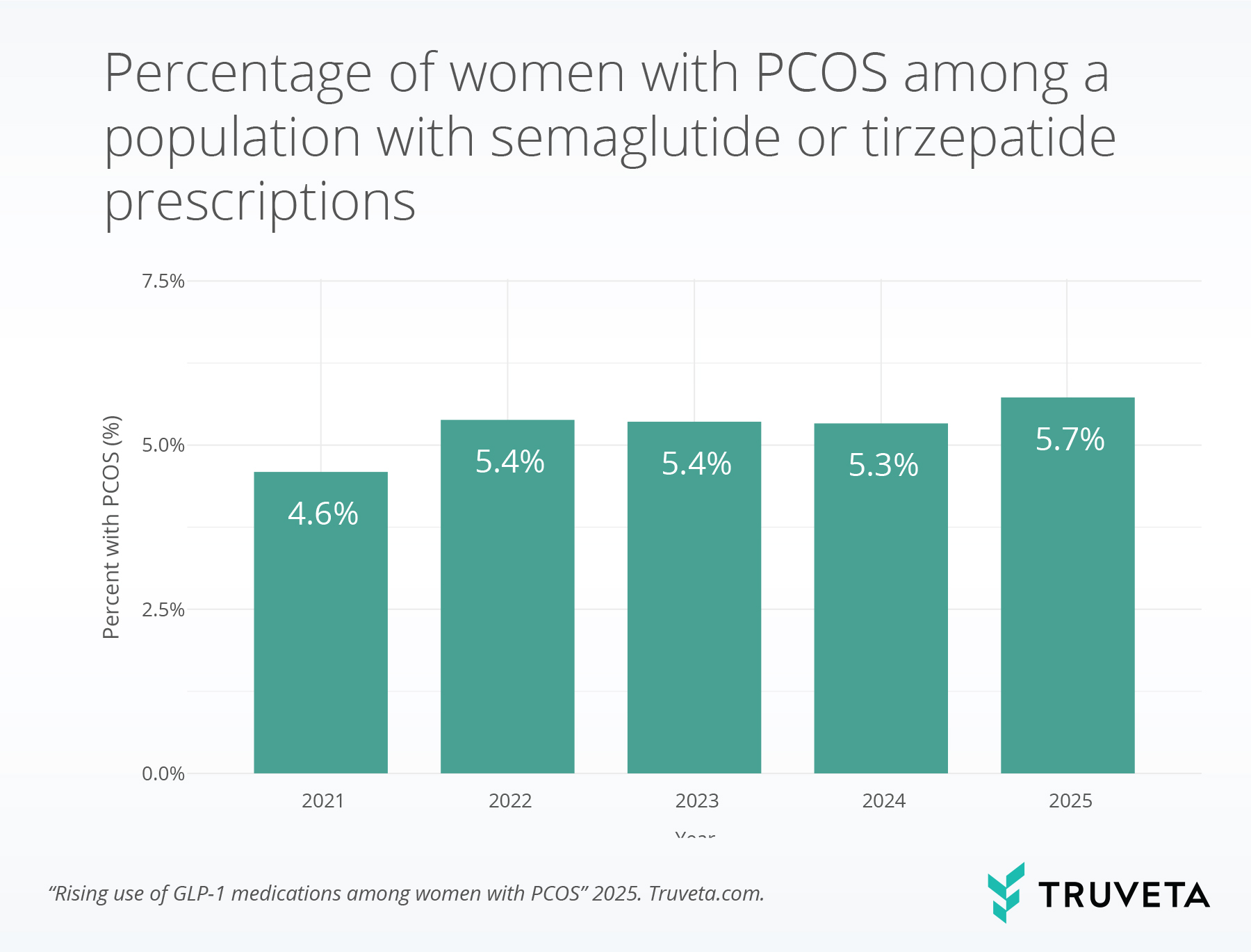
- Among women prescribed a semaglutide or tirzepatide medication, the percentage with a PCOS diagnosis increased from 4.6% in 2021 to 5.7% in 2025, a 23.9% relative increase.
- Nearly all PCOS patients prescribed semaglutide or tirzepatide also had obesity or type 2 diabetes, suggesting use remains primarily tied to metabolic indications, though broader interest in PCOS benefits is growing within scientific literature.
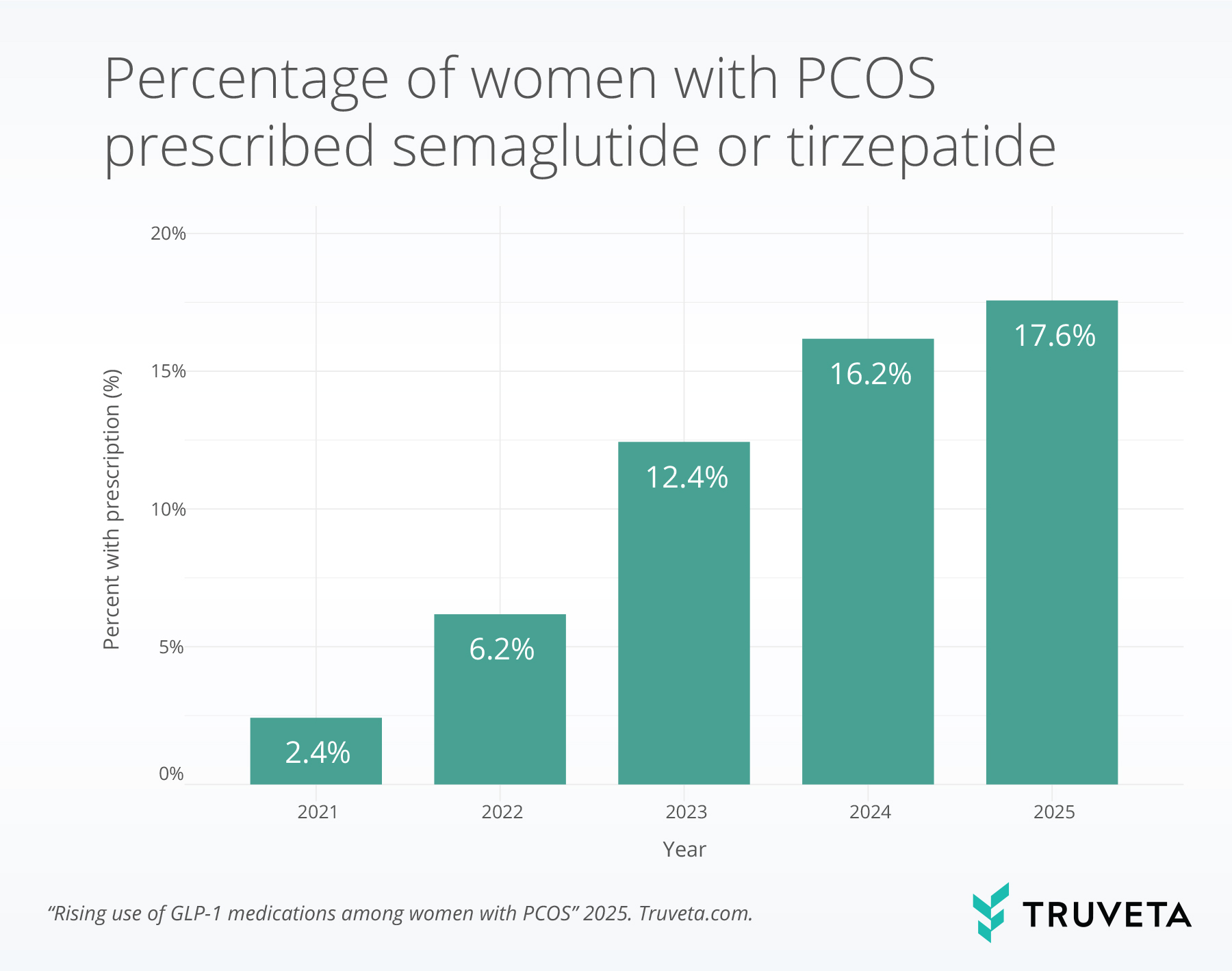
- Among women with PCOS, semaglutide or tirzepatide prescribing increased from 2.4% in 2021 to 17.6% in 2025, a more than 7-fold increase.
Polycystic ovary syndrome (PCOS) is a condition that affects hormonal balance, affecting 6-10% of reproductive-aged women (1). Patients with PCOS experience excess production of androgens, which disrupts ovulation and leads to irregular menstrual cycles, infertility, acne, excess hair growth, and weight gain (1, 2). PCOS is also linked to other health conditions, including metabolic issues such as insulin resistance and type 2 diabetes (3).
GLP-1 receptor agonists (GLP-1 RAs) are primarily used to treat obesity and type 2 diabetes; however, growing evidence suggests that they may also provide benefits for other conditions, including PCOS (4, 5). Although not approved specifically for PCOS, GLP-1 RA use in women with PCOS has been associated with improved blood glucose control and weight loss, as well as improvements in menstrual regularity and a decrease in ovarian cysts (4, 6–8). Weight management and blood glucose control are central to PCOS because the condition is strongly associated with insulin resistance (3, 7). Elevated insulin levels can drive excess androgen production, which in turn contributes to irregular ovulation and other symptoms of PCOS (9). As such, reducing insulin resistance and supporting healthy weight are important goals in the overall treatment and management of PCOS (1, 3).
In addition to effects on weight and blood glucose control, early laboratory studies in mice suggest that GLP-1 RAs might work through additional biological mechanisms (10, 11). For instance, GLP-1 RAs appear to reduce inflammation in the ovaries and may help lower androgen levels (10, 12), indicating potential broader effects on reproductive and metabolic health.
Given these emerging connections and in partnership with Reuters, we wanted to better understand the real-world overlap between people using GLP-1 RA medications and those diagnosed with PCOS. Specifically, our research explores two key questions over time:
- Among patients prescribed a semaglutide or tirzepatide medication, how many also have a diagnosis of PCOS?
- Among patients with a diagnosis of PCOS, how many were prescribed semaglutide or tirzepatide?
By examining these relationships, we aim to better understand how semaglutide and tirzepatide medications are being used among patients with PCOS.
Methods
We looked at a subset of the Truveta Data from January 2021 to September 2025 to identify two patient cohorts. The first cohort included female patients who received a semaglutide or tirzepatide prescription during the study period. The second cohort included patients with a diagnosis of polycystic ovary syndrome (PCOS) recorded during or before the study period.
For each calendar year, we calculated rates describing the overlap between semaglutide or tirzepatide prescriptions and PCOS diagnoses:
- Among female patients who received a semaglutide or tirzepatide prescription in a given year, we calculated the proportion who had a recorded PCOS diagnosis in or before that year.
- Among patients with a prior PCOS diagnosis, who also had a medical encounter during that year, we calculated the proportion who received a semaglutide or tirzepatide prescription in that year.
This approach allowed us to evaluate annual trends among these two populations. You can view the entire study directly within Truveta Studio.
Results
PCOS among females with semaglutide and tirzepatide prescriptions
Between 2021 and 2025, 1,738,008 female patients received a prescription for semaglutide or tirzepatide. Of these patients, 90,210 (5.2%) had a diagnosis of PCOS. Over the four-year period, the percentage of female patients with a semaglutide or tirzepatide prescription who also had PCOS increased from 4.6% in 2021 to 5.7% in 2025, representing a 23.9% relative increase.
Among patients prescribed semaglutide or tirzepatide, 97.8% of those with PCOS also had obesity and/or type 2 diabetes. In comparison, 88.2% of those without PCOS with a semaglutide or tirzepatide prescription had obesity and/or diabetes.
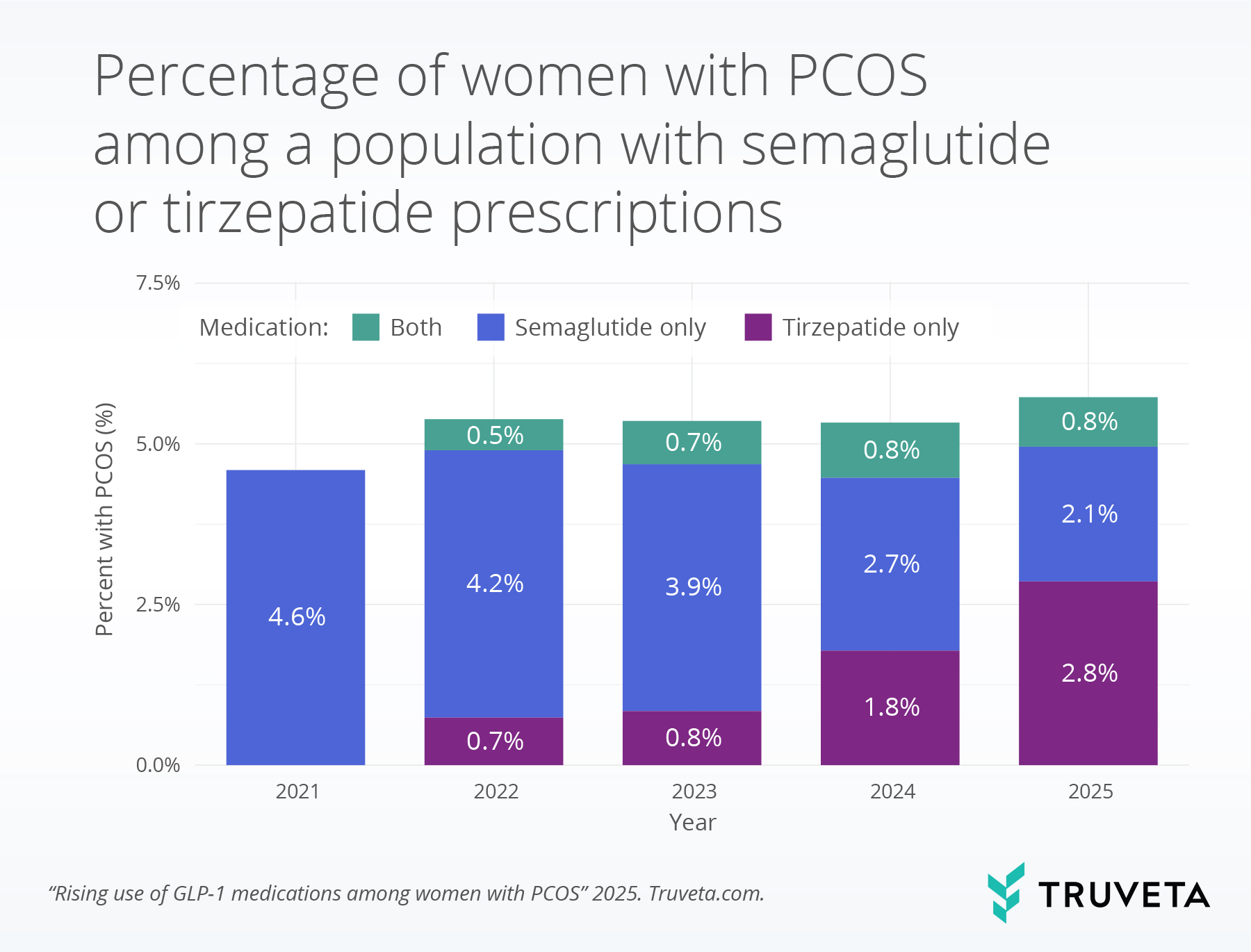
Among patients with PCOS who had a semaglutide or tirzepatide prescription, the percentage of patients prescribed tirzepatide increased, while semaglutide use declined over time. Specifically, tirzepatide prescriptions increased from 0.7% in 2022 to 2.8% in 2025, whereas semaglutide prescriptions decreased from 4.2% in 2022 to 2.1% in 2025.
Prescriptions for semaglutide and tirzepatide among patients with PCOS
Between 2021 and 2025, 445,258 patients with PCOS had at least one medical encounter, of whom 90,206 (20.3%) received a semaglutide or tirzepatide prescription during the study period. The proportion of patients with PCOS who were prescribed semaglutide or tirzepatide increased from 2.4% in 2021 to 17.6% in 2025, representing a 637.5% relative increase.
Discussion
In this analysis, the proportion of semaglutide or tirzepatide prescriptions associated with a diagnosis of PCOS increased modestly between 2021 and 2025, from 4.6% to 5.7%. Nearly all patients with PCOS who were prescribed semaglutide or tirzepatide also had obesity and/or diabetes, conditions for which these medications have FDA-approved indications. This observed increase may reflect the high prevalence of metabolic comorbidities among patients with PCOS, rather than expanded prescribing of GLP-1s specifically for PCOS.
Metabolic conditions are very common among people with PCOS, including insulin resistance, impaired glucose tolerance, type 2 diabetes, dyslipidemia, and obesity (3). Combined oral contraceptive pills are often used to manage menstrual irregularity and androgen-related symptoms, while metformin is commonly prescribed to address insulin resistance and other metabolic issues (13). GLP-1 RAs, such as semaglutide and tirzepatide, may also help improve metabolic health in patients with PCOS by promoting weight loss, improving insulin sensitivity, and reducing blood glucose levels (4, 6, 14). The high rates of obesity and diabetes among PCOS patients receiving semaglutide and tirzepatide in this study are consistent with these metabolic features and suggest that GLP-1 RA therapy may play an expanding role in addressing the metabolic aspects of PCOS.
Tirzepatide use increased over time, consistent with broader population trends (15). This rise may relate to emerging evidence of its metabolic efficacy and weight loss benefits, which could be influencing prescribing patterns among patients with PCOS (16).
The proportion of patients with PCOS who were prescribed semaglutide or tirzepatide increased more than 7-fold over the study period. This trend aligns with broader increases in GLP-1 RA utilization across the general population during the same period (15). Thus, the growth in GLP-1 RA prescribing among patients with PCOS may reflect a combination of general expansion in GLP-1 RA use and the high prevalence of metabolic indications in this population.
Collectively, these findings highlight that patients with PCOS frequently meet traditional criteria for GLP-1 RA therapy and show gradual increase among GLP-1 RA users. Further research is warranted to determine whether GLP-1 RAs are being prescribed primarily for metabolic indications in this group or whether clinical use is expanding to include PCOS-related symptoms and outcomes.
This study has several limitations. First, PCOS was identified based on the presence of at least one diagnosis code, without requiring that it appear as a primary diagnosis or be confirmed across multiple encounters. As a result, some patients may have been misclassified if PCOS was listed as a secondary or historical condition rather than an active clinical diagnosis. Second, this analysis focused exclusively on a subset of GLP-1 RA therapies (specifically semaglutide and tirzepatide) and did not account for other common PCOS treatments, such as metformin, combined oral contraceptives, or anti-androgen therapies. As a result, the findings do not reflect the broader treatment landscape for PCOS or how GLP-1 RA use compares with these standard therapies.
These are preliminary research findings and not peer reviewed. Data are constantly changing and updating. These findings are consistent with data accessed on October 6, 2025.
Citations
- K. Walter, What Is Polycystic Ovary Syndrome? JAMA 327, 294 (2022).
- S. Singh, N. Pal, S. Shubham, D. K. Sarma, V. Verma, F. Marotta, M. Kumar, Polycystic Ovary Syndrome: Etiology, Current Management, and Future Therapeutics. J Clin Med 12, 1454 (2023).
- Y. M. Jeanes, S. Reeves, Metabolic consequences of obesity and insulin resistance in polycystic ovary syndrome: diagnostic and methodological challenges. Nutrition research reviews 30, 97–105 (2017).
- S. Rahim, J. Pergolizzi, The Potential Role of Glucagon-Like Peptide-1 (GLP-1) Agonists for Polycystic Ovary Syndrome. Cureus, doi: 10.7759/cureus.77998 (2025).
- M. Siamashvili, S. N. Davis, Update on the effects of GLP-1 receptor agonists for the treatment of polycystic ovary syndrome. Expert Review of Clinical Pharmacology 14, 1081–1089 (2021).
- J. Ferdous, M. Hossain, M. Faika, M. Begum, S. Mahjabeen, I. Jahan, M. Khan, M. Hossain, Role of Tirzepatide in Obesity Management Among Women with Polycystic Ovary Syndrome. IJDE 10, 37–44 (2025).
- C. Xing, C. Li, B. He, Insulin sensitizers for improving the endocrine and metabolic profile in overweight women with PCOS. The Journal of Clinical Endocrinology & Metabolism 105, 2950–2963 (2020).
- K. Bednarz, K. Kowalczyk, M. Cwynar, D. Czapla, W. Czarkowski, D. Kmita, A. Nowak, P. Madej, The Role of Glp-1 Receptor Agonists in Insulin Resistance with Concomitant Obesity Treatment in Polycystic Ovary Syndrome. International Journal of Molecular Sciences 23, 4334 (2022).
- H. Ding, J. Zhang, F. Zhang, S. Zhang, X. Chen, W. Liang, Q. Xie, Resistance to the insulin and elevated level of androgen: A major cause of polycystic ovary syndrome. Frontiers in endocrinology 12, 741764 (2021).
- M. Liu, S. Guo, X. Li, Y. Tian, Y. Yu, L. Tang, Q. Sun, T. Zhang, M. Fan, L. Zhang, Y. Xu, J. An, X. Gao, L. Han, L. Zhang, Semaglutide Alleviates Ovary Inflammation via the AMPK/SIRT1/NF‑κB Signaling Pathway in Polycystic Ovary Syndrome Mice. DDDT Volume 18, 3925–3938 (2024).
- S. Guo, X. Li, M. Liu, M. Feng, X. Wang, H. Xue, L. Zhang, Semaglutide Alleviates Ovarian Oxidative Stress and Autophagy via the PI3K/AKT/mTOR Pathway in Mice with Polycystic Ovary Syndrome. DDDT Volume 19, 4297–4310 (2025).
- Y. Zhang, Y. Lin, G. Li, Y. Yuan, X. Wang, N. Li, C. Xiong, Y. Yang, Y. Ma, Z. Zhang, Glucagon-like peptide-1 receptor agonists decrease hyperinsulinemia and hyperandrogenemia in dehydroepiandrosterone-induced polycystic ovary syndrome mice and are associated with mitigating inflammation and inducing browning of white adipose tissue. Biology of Reproduction 108, 945–959 (2023).
- T. Williams, R. Mortada, S. Porter, Diagnosis and treatment of polycystic ovary syndrome. American family physician 94, 106–113 (2016).
- H. Chen, X. Lei, Z. Yang, Y. Xu, D. Liu, C. Wang, H. Du, Effects of combined metformin and semaglutide therapy on body weight, metabolic parameters, and reproductive outcomes in overweight/obese women with polycystic ovary syndrome: a prospective, randomized, controlled, open-label clinical trial. Reprod Biol Endocrinol 23, 108 (2025).
- S. Gratzl, B. M. G. Cartwright, P. J. Rodriguez, K. Gilbert, D. Do, N. Masters, N. L. Stucky, Monitoring Report: GLP-1 RA Prescribing Trends – June 2025 Data. medRxiv, 2025.03.06.25323524 (2025).

