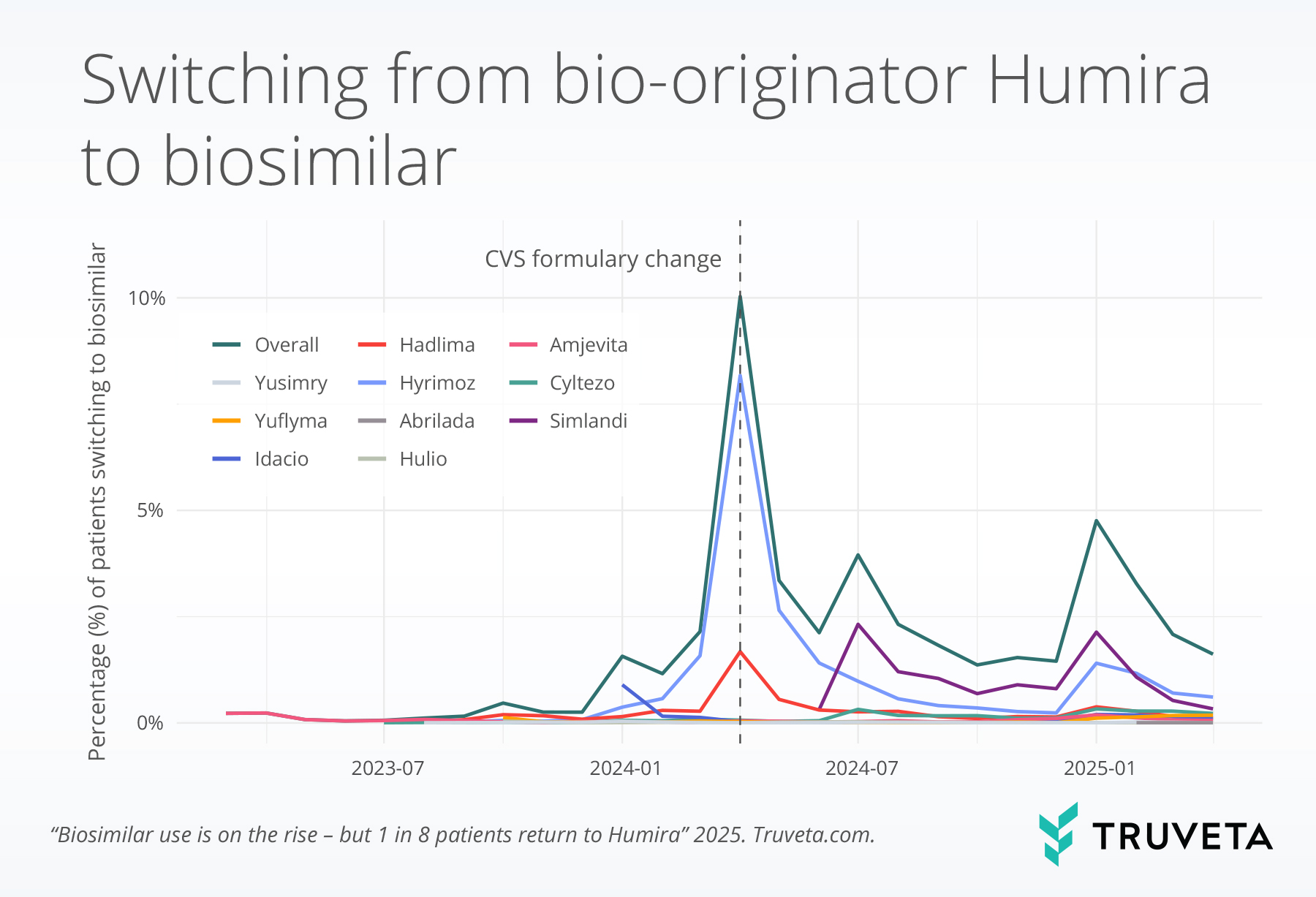
- Switching from bio-originator Humira to a biosimilar peaked in April 2024 – the month an important formulary change took effect – and remains higher than 2023, suggesting growing adoption of biosimilars.
- More than 1 in 8 patients who initiated a biosimilar switched back to bio-originator Humira; switching back was more common among older adults, women, and patients with rheumatoid arthritis and ankylosing spondylitis.
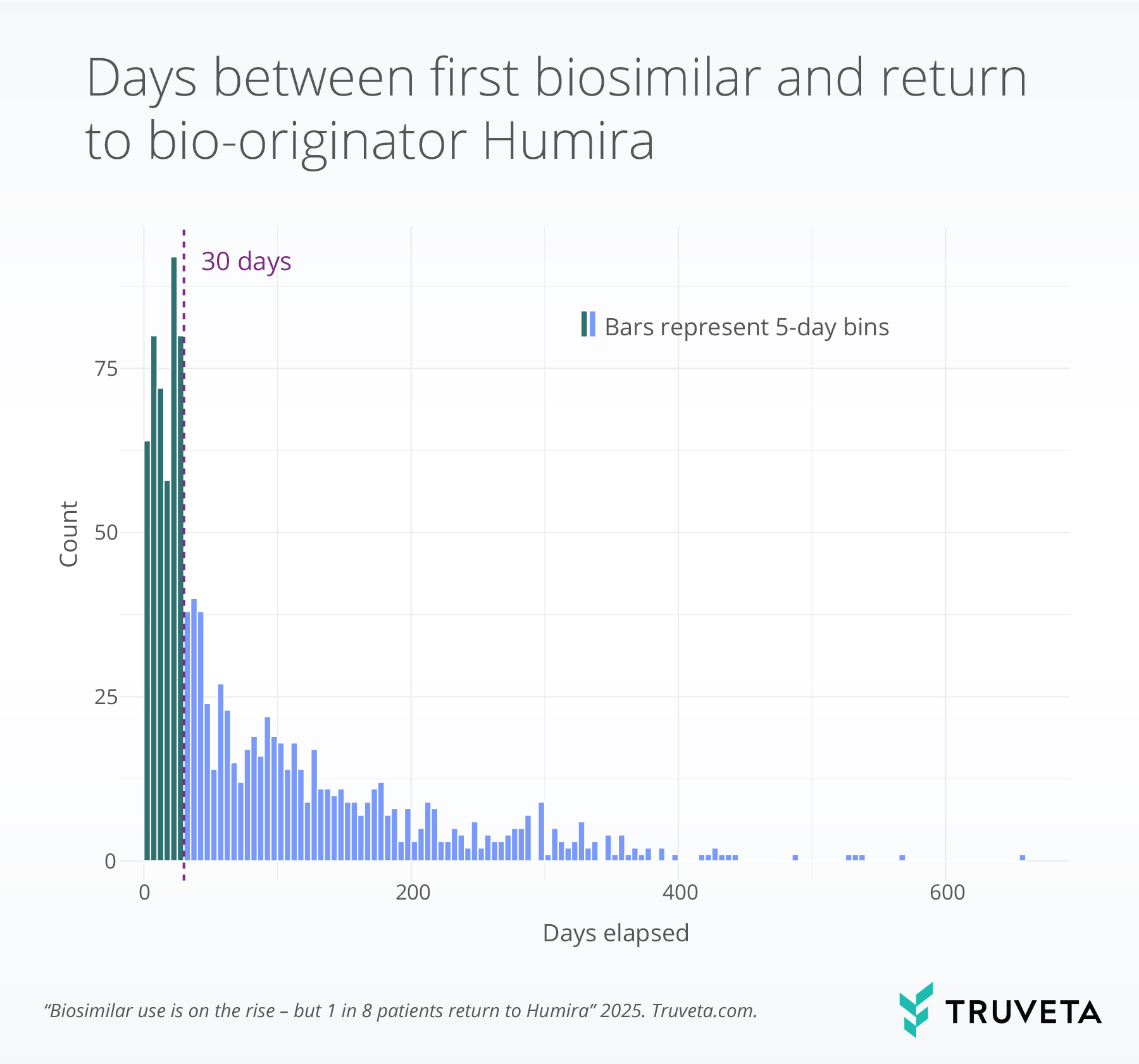
- Among patients who switched back to bio-originator Humira, more than one third did so within 30 days, suggesting early dissatisfaction, adverse events, or resistance to switching.
Adalimumab (bio-originator Humira) is a biologic medication used to treat a range of chronic autoimmune conditions, including rheumatoid arthritis, psoriatic arthritis, ankylosing spondylitis, psoriasis, Crohn’s disease, and ulcerative colitis. Since its approval in the early 2000s, bio-originator Humira has been the top-selling medication globally, with a prolonged period of market exclusivity in the United States contributing to substantial price increases over time (1, 2).
In January 2023, the first adalimumab biosimilar — highly similar, but not identical versions of the original biologic — entered the market, with the goal of reducing costs and improving patient access (3–5). Biosimilars are estimated to be priced between 5% to 85% lower than Humira (6, 7), and undergo rigorous regulatory evaluation to demonstrate no clinically meaningful differences in safety or efficacy compared to the reference biologic. Clinical trials and meta-analyses examining the ten Humira biosimilars currently approved in the US have showed similar outcomes and side effects to bio-originator Humira (8–12). However, initial market data indicated that uptake of biosimilar was slow despite lower prices (13).
In January 2024, CVS Health, one of the largest pharmacy benefit managers in the US, announced changes to promote biosimilar adoption. The company removed the bio-originator Humira from its formulary for certain patients and designated the biosimilar Hyrimoz as the preferred option starting in April 2024. Our initial analysis – with data through April 2024 – showed an increase in switching following this announcement, primarily driven by switching to Hyrimoz.
While this initial trend reflects growing biosimilar initiation, it also underscores the need to understand how patients respond to biosimilar medications in real-world settings, including whether they remain on the biosimilar. Surveys show many patients are unfamiliar with biosimilars and often express concerns about safety and effectiveness (14–16). Negative perceptions may contribute not only to hesitancy in switching but also to discontinuation after switching (16, 17). Studies of other biologic drug classes have found that patients who discontinue a biosimilar often return to the originator (18–20), even though clinical trials show no meaningful differences (19).
Switching-back patterns may reflect real-world experiences—driven by perceptions, symptoms, or clinical uncertainty—that diverge from expectations based on clinical trial findings (16). Building on our previous analysis of biosimilar switching rates, this follow-up report extends the evaluation period and investigates patterns of patients who switched back to bio-originator Humira after starting a biosimilar. Understanding the frequency and timing of these switchbacks is critical for assessing real-world biosimilar adoption and identifying potential barriers to sustained biosimilar use.
Methods
A subset of Truveta Data was used to define a cohort of patients potentially eligible for biosimilar Humira. Patients were included if they had an outpatient office or telehealth visit on or after January 31, 2023 (the date biosimilars became available), at least two prescription fills for bio-originator Humira in the 12 months preceding the visit, and no prior biosimilar dispense recorded before the encounter.
You can review the entire report—including data definitions and code—directly in Truveta Studio.
Switching from bio-originator Humira to biosimilar
Switching from bio-originator Humira to a biosimilar was defined by dispensing of a biosimilar version among patients previously dispensed bio-originator Humira.
Monthly switching rates were calculated as the number of patients with a first-time biosimilar dispense in a given month divided by the number of patients eligible to switch in that month (i.e., those with an encounter during that month who had not yet switched), reported as percentage of eligible patients.
Switching back to bio-originator Humira
Switching back to bio-originator Humira was defined by a subsequent dispense of bio-originator Humira after a biosimilar dispense. The proportion of these patients who switched back was calculated overall and stratified by patient characteristics including sex, race, age group, and condition (e.g., rheumatoid arthritis, psoriasis). To ensure observability of switching, patients were required to have at least one outpatient or telehealth encounter following their first biosimilar dispense.
Timing of returning to bio-originator Humira
Among patients who switched back to bio-originator Humira, we assessed the duration between the initial biosimilar dispense date and the subsequent bio-originator Humira dispense date. As bio-originator Humira and its biosimilars are typically dosed biweekly and dispensed in quantities covering approximately 30 days, we used a 30-day interval to define early switchbacks.
Results
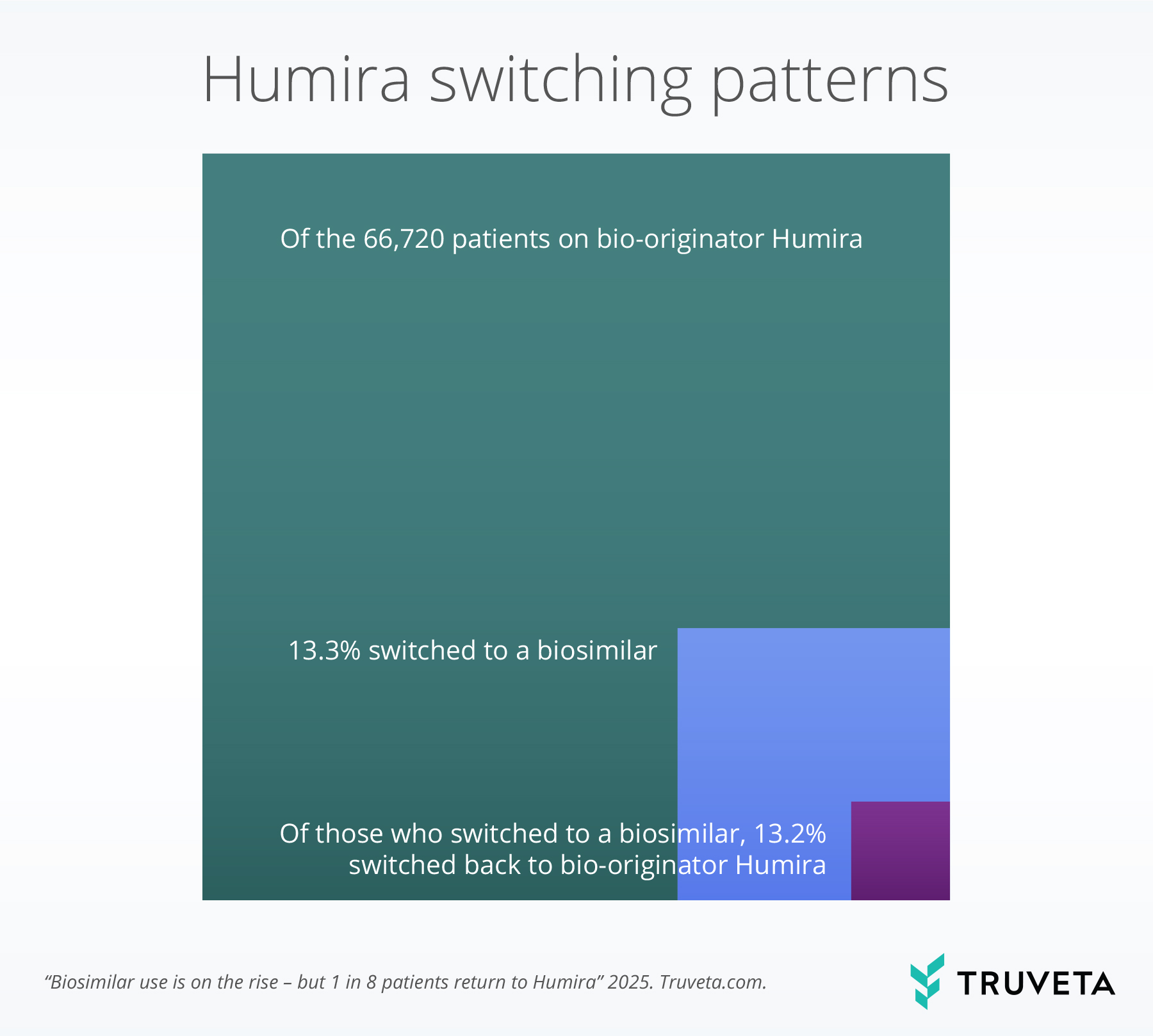
Of 66,720 eligible patients on bio-originator Humira, 13.3% (8,876) had evidence of switching to a biosimilar by April 2025.
Switching from bio-originator Humira to biosimilar
Switching to a biosimilar was minimal in 2023 but increased sharply after January 2024. In December 2023, 0.3% of patients switched to a biosimilar.
In January 2024, this increased to 1.6% of patients; by April 2024, it reached 10.0% of patients. Afterward, switching declined to 4.8% in January 2025 and further decreased to 1.6% in April 2025.
Across the full time period from January 2023 to April 2025, Hyrimoz accounted for the largest share of biosimilar switches (47.3%), followed by Simlandi (23.8%), and Hadlima (12.5%).
Between January 1 and April 30, 2024—a period during which CVS announced and implemented its biosimilar formulary change—Hyrimoz accounted for 71.9% of all biosimilar switches from bio-originator Humira.
From May 2024 through April 2025, the distribution shifted: Simlandi became the most common biosimilar, representing 38.0% of switches, followed closely by Hyrimoz (36.7%).
Switching back to bio-originator Humira
Of the 8,594 patients who switched to a biosimilar and had adequate follow-up, 13.2% (1,138) had evidence of switching back to bio-originator Humira.
This means for every 100 people on bio-originator Humira, 13 switched to a biosimilar, and 2 switched to a biosimilar and then switched back to bio-originator Humira.
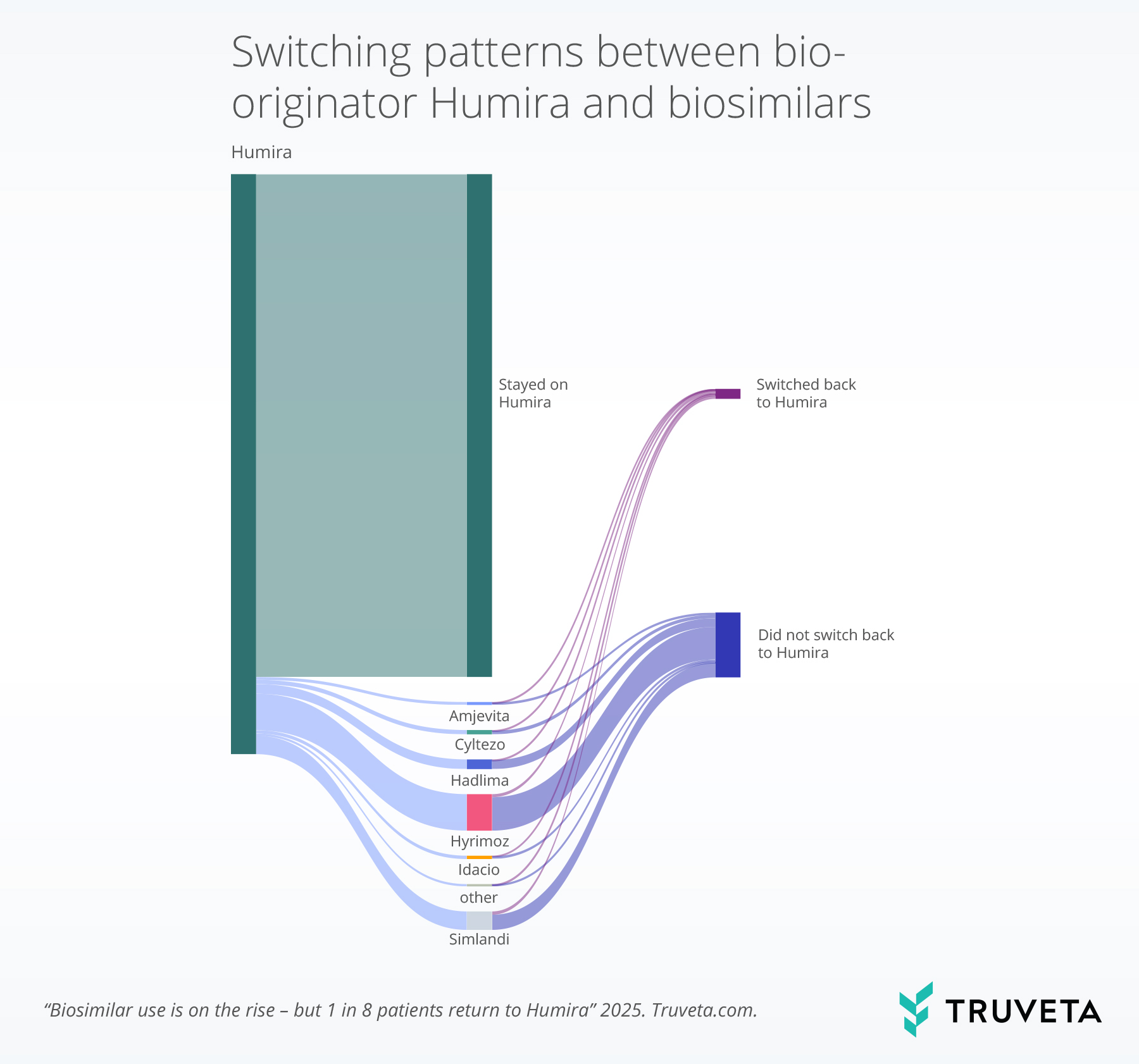
The below flow diagram or sankey chart shows how patients who started on bio-originator Humira moved through different treatment paths.
The left green bar shows initial bio-originator Humira use, and the diagram tracks whether patients stayed on bio-originator Humira (same green color) or switched to a specific biosimilar.
The final section shows whether patients who switched remained on the biosimilar (blue) or returned to bio-originator Humira (purple).
The line thickness reflects the number of patients. Biosimilar medications with greater than 200 patients were plotted; for these drugs the percent of patient who switched back to bio-originator Humira ranged from 7.8%-18.5%.
Female patients were more likely to switch back to bio-originator Humira than male patients (14.3% vs. 11.4%).
Additionally, patients over 65 years of age switched back at higher rates (20.2%) compared to younger age groups [18–34 years (11.7%), 25–49 years (12.2%), and 50–64 years (13.0%)].
Patients with rheumatoid arthritis and ankylosing spondylitis had slightly higher rates of switching back than those with other conditions studied.
No notable differences were seen by race or urbanicity.
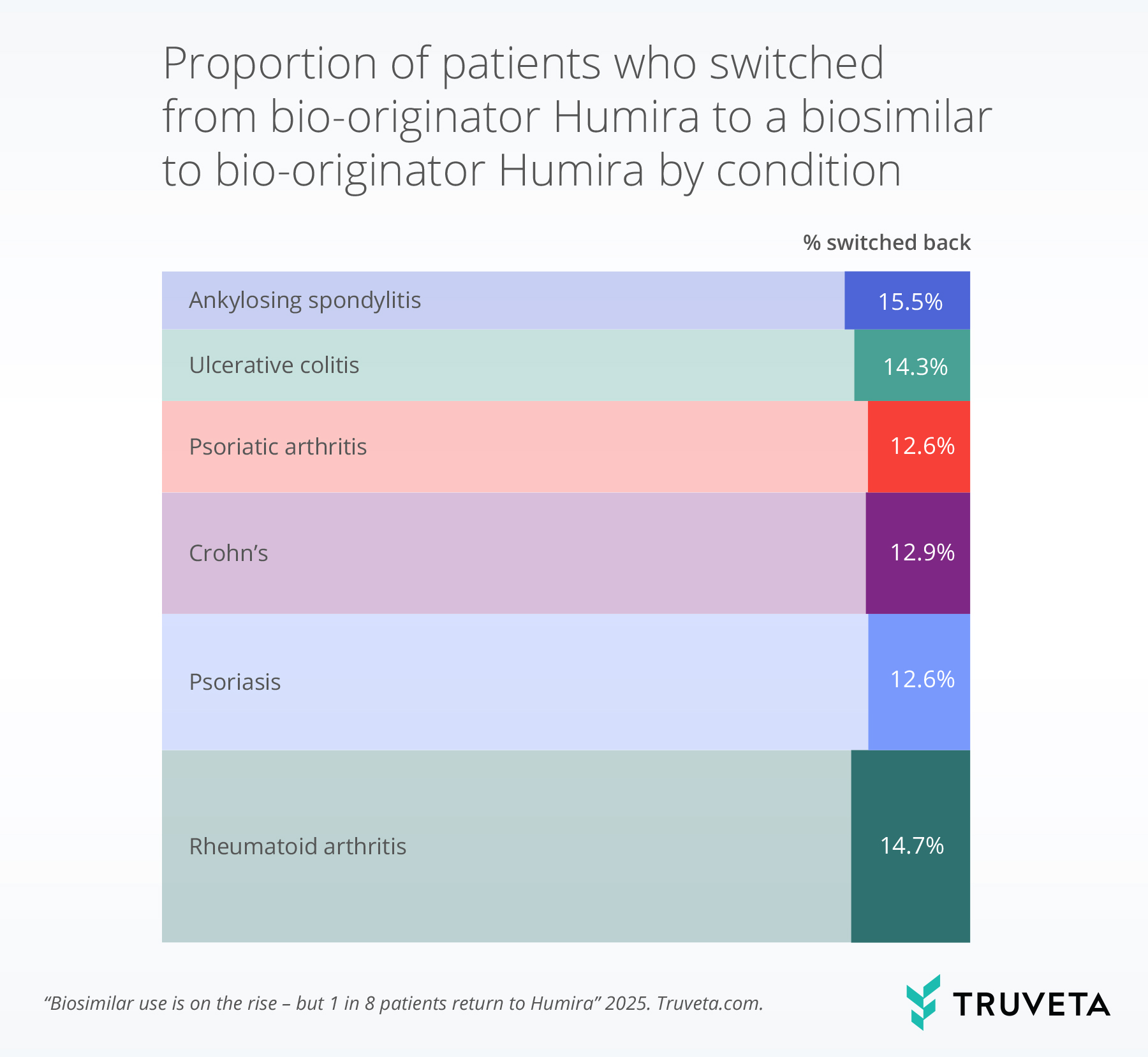
The bar width indicates the relative volume of the medication prescribed for that condition. The more saturated portion of each bar represents the percentage of patients within that condition who switched back.
Timing of returning to bio-originator Humira
Overall, 39.7% of patients who returned to bio-originator Humira did so within 30 days of initiating a biosimilar.
Early switchbacks were more common among older adults, with patients aged 65 and older more likely to return to bio-originator Humira within 30 days compared to younger age groups.
Additionally, older patients showed higher rates of early switch backs compared to younger patients, with 51.8% of those aged 65 and older switching back early versus 41.9%, 40.1%, and 35.1% for the 18–34, 25–49, and 50–64 age groups, respectively.
Also, patients residing in rural areas (43.9%) were more likely to switch back to bio-originator Humira within 30 days than those in urban areas (36.3%).
No notable differences in early switchbacks were observed by patient condition, sex, or race.
Discussion
Switching from bio-originator Humira to a biosimilar peaked in April 2024, which coincided with CVS no longer carrying bio-originator Humira and instead recommending Hyrimoz as the preferred option. Although the rate of switching to a biosimilar hasn’t climbed higher since then, it has stayed well above the levels seen when biosimilars first became available in 2023. This increase in switching to a biosimilar may reflect changes in insurer policies, growing trust among providers and patients in biosimilars, or broader shifts in the drug market. For example, some insurers announced that beginning in January 1, 2025, biosimilars such as Simlandi, Idacio, and Amjevita would become the preferred alternatives to bio-originator Humira (21–23).
Despite the rise in biosimilar use, more than one in eight patients who switched eventually returned to bio-originator Humira, indicating that initial switches may not always be sustained despite lower cost. Switching back was more common among older adults, women, and people with rheumatoid arthritis or ankylosing spondylitis suggesting that patient characteristics may influence tolerance or satisfaction with biosimilar medication. Notably, nearly 40% of patients who returned to bio-originator Humira did so within 30 days of initiating a biosimilar, suggesting early dissatisfaction, adverse reactions. Given that many patients switched back before completing a typical Humira dispense, this pattern may also reflect fear or uncertainty about switching to a biosimilar.
We also found that patients living in rural areas and adults over the age of 65 were more likely to switch back within 30 days. For rural patients, this might be because their local pharmacy didn’t have Humira in stock, so they were given a biosimilar by default—not as a deliberate switch. For older adults, earlier switching-back rates might be related to increased caution to medication changes, more complex health conditions, or their doctors wanting to keep them on a treatment that’s already working well.
Our finding that 13.2% of patients switched back to bio-originator Humira is similar to a meta-analysis of 62 studies in European populations, which reported that 14% of patients using biosimilars of infliximab and etanercept switched back to the original biologics within one year (24). Our report only included US patients, where rules about when biosimilars enter the market and cost structures differ from other countries. Additionally, unlike many previous studies, we specifically examined switching patterns related to bio-originator Humira and its biosimilars.
The switching patterns discussed in this report may reflect broader hesitations among patients and providers. Patients and providers alike report feeling hesitant to switch from a medication that is working well to a biosimilar (25–27). Surveys indicate that few patients have heard of biosimilars, and among those who have, many express concerns about giving up efficacy and safety in exchange for a lower-cost alternative (14, 15, 28). Prior studies have suggested that a patient’s negative expectations about switching to a biosimilar may contribute to discontinuation through a perceived loss of effectiveness or worsening of symptoms, even when clinical evidence indicates no meaningful difference in safety or efficacy between the biosimilar and the originator (16, 17, 29). More research is needed to better understand the reasons behind patients switching back to original biologics (30).
Several limitations should be considered when interpreting our findings. First, our analysis was limited to first-time switches from bio-originator Humira to a biosimilar and we did not assess subsequent switches between different biosimilars, initiation of a biosimilar without a prior bio-originator Humira dispense, or medication discontinuation. Second, we did not assess reasons for switching to or back to bio-originator Humira. Third, we did not account for differences in timing of biosimilar approvals or indications, which could affect prescribing patterns.
These results point to the need for ongoing research to better understand the factors driving patients to switch back to originator biologics after initiating biosimilars. While switching back does occur, it represents a relatively small proportion of patients. Studies based on real-world data can help capture a wider range of patient experiences and clinical scenarios than traditional trials, including factors that may influence treatment satisfaction or tolerability. Such insights help illuminate how treatment decisions are made in practice, where shared decision-making between providers and patients plays a central role. By addressing the concerns of both patients and providers, healthcare systems can encourage more sustained use of these cost-saving alternatives without compromising treatment quality.
These are preliminary research findings and not peer reviewed. Data are constantly changing and updating. These findings are consistent with data accessed on May 19, 2025.
You can review the entire report—including data definitions and code—directly in Truveta Studio.
Citations
- J. Gibbons, M. Laber, C. Bennett, Humira: The First $20 Billion Drug. 29 (2023).
- S. R. Dickson, N. Gabriel, I. Hernandez, Contextualizing the Price of Biosimilar Adalimumab Based on Historical Rebates for the Original Formulation of Branded Adalimumab. JAMA Network Open 6, e2323398 (2023).
- A. W. Mulcahy, J. P. Hlavka, S. R. Case, Biosimilar Cost Savings in the United States. Rand Health Q 7, 3 (2018).
- C. C. Lee, M. Najafzadeh, A. S. Kesselheim, A. Sarpatwari, Cost to Medicare of Delayed Adalimumab Biosimilar Availability. Clin Pharma and Therapeutics 110, 1050–1056 (2021).
- L. Joszt, The Wait Is Over: 8 Adalimumab Biosimilars Launching in the US in July, AJMC (2023). https://www.ajmc.com/view/the-wait-is-over-8-adalimumab-biosimilars-launching-in-the-us-in-july.
- R. Volansky, Drug costs and nocebo risks: Navigating the Humira ‘biosimilar boom’ 1 year later, Healio Rheumatology (2024). https://www.healio.com/news/rheumatology/20240208/drug-costs-and-nocebo-risks-navigating-the-humira-biosimilar-boom-1-year-later.
- S. R. Dickson, N. Gabriel, I. Hernandez, Contextualizing the price of biosimilar adalimumab based on historical rebates for the original formulation of branded adalimumab. JAMA Network Open 6, e2323398–e2323398 (2023).
- X. Lu, R. Hu, L. Peng, M. Liu, Z. Sun, Efficacy and Safety of Adalimumab Biosimilars: Current Critical Clinical Data in Rheumatoid Arthritis. Front. Immunol. 12 (2021).
- J. E. McClellan, S. Ómarsdóttir, N. Roy, V. Berger, C. Michel, F. Berti, The totality of evidence approach in the development of AVT02 (adalimumab), a biosimilar to Humira. Therapeutic Advances in Chronic Disease 15, 20406223231223286 (2024).
- B. de O. Ascef, M. O. Almeida, A. C. de Medeiros-Ribeiro, D. C. Oliveira de Andrade, H. A. de Oliveira Junior, P. C. de Soárez, Therapeutic Equivalence of Biosimilar and Reference Biologic Drugs in Rheumatoid Arthritis: A Systematic Review and Meta-analysis. JAMA Network Open 6, e2315872 (2023).
- B. de Oliveira Ascef, M. O. Almeida, A. C. de Medeiros-Ribeiro, D. C. O. de Andrade, H. A. de Oliveira Junior, P. C. de Soarez, Therapeutic equivalence of biosimilar and reference biologic drugs in rheumatoid arthritis: A systematic review and meta-analysis. JAMA Network Open 6, e2315872–e2315872 (2023).
- T. W. J. Huizinga, Y. Torii, R. Muniz, Adalimumab Biosimilars in the Treatment of Rheumatoid Arthritis: A Systematic Review of the Evidence for Biosimilarity. Rheumatol Ther 8, 41–61 (2021).
- 6 min read | Annalee Armstrong, Humira Biosimilars Gain Ground as Doctors Adjust and New Therapies Rise, BioSpace (2025). https://www.biospace.com/business/humira-biosimilars-gain-ground-as-doctors-adjust-and-new-therapies-rise.
- D. Pineles, A. Arsuaga, L. B. Malter, B. P. Bosworth, D. P. Hudesman, S. Chang, Patient Perceptions Regarding the Use of Biosimilars in Inflammatory Bowel Disease: 750. Official journal of the American College of Gastroenterology | ACG 112, S418 (2017).
- L. Peyrin-Biroulet, S. Lönnfors, X. Roblin, S. Danese, L. Avedano, Patient Perspectives on Biosimilars: A Survey by the European Federation of Crohn’s and Ulcerative Colitis Associations. Journal of Crohn’s and Colitis 11, 128–133 (2017).
- M. F. Rezk, B. Pieper, Treatment Outcomes with Biosimilars: Be Aware of the Nocebo Effect. Rheumatol Ther 4, 209–218 (2017).
- C. Gasteiger, M. Lobo, N. Dalbeth, K. J. Petrie, Patients’ beliefs and behaviours are associated with perceptions of safety and concerns in a hypothetical biosimilar switch. Rheumatol Int 41, 163–171 (2021).
- T. Fitzgerald, R. Melsheimer, M.-H. Lafeuille, P. Lefebvre, L. Morrison, K. Woodruff, I. Lin, B. Emond, Switching and Discontinuation Patterns Among Patients Stable on Originator Infliximab Who Switched to an Infliximab Biosimilar or Remained on Originator Infliximab. BTT Volume 15, 1–15 (2021).
- R. Fleischmann, V. Jairath, E. Mysler, D. Nicholls, P. Declerck, Nonmedical Switching From Originators to Biosimilars: Does the Nocebo Effect Explain Treatment Failures and Adverse Events in Rheumatology and Gastroenterology? Rheumatol Ther 7, 35–64 (2020).
- N. W. Boone, L. Liu, M. J. Romberg-Camps, L. Duijsens, C. Houwen, P. H. M. Van Der Kuy, R. Janknegt, R. Peeters, R. B. M. Landewé, B. Winkens, A. A. Van Bodegraven, The nocebo effect challenges the non-medical infliximab switch in practice. Eur J Clin Pharmacol 74, 655–661 (2018).
- Effective January 1, 2025, IBX is removing Humira® (adalimumab) from its Value, Select, and Premium formularies and switching to a biosimilar, Independence Provider News Center. https://provcomm.ibx.com/pnc-ibc/news/Pages/Effective-January-1-2025-IBX-is-removing-Humira-adalimumab-from-its-Value-Select-and-Premium-formularies.aspx.
- Optum Rx Switches Up Humira Biosimilar Coverage for 2025, Managed Healthcare Executive (2024). https://www.managedhealthcareexecutive.com/view/optum-rx-switches-up-humira-biosimilar-coverage-for-2025.
- Blue Cross Blue Shield Blue Care Network of Michigan, Simlandi will be our preferred adalimumab product, starting Jan. 1 (2024).
- Y. Liu, M. Skup, M. Yang, C. Z. Qi, E. Q. Wu, Discontinuation and Switchback After Non-Medical Switching from Originator Tumor Necrosis Factor Alpha (TNF) Inhibitors to Biosimilars: A Meta-Analysis of Real-World Studies from 2012 to 2018. Adv Ther 39, 3711–3734 (2022).
- M. S. Niaz, S. Suhail, U. Mahmood, Awareness and Knowledge of Biosimilars Among Rheumatologists and Patients. Journal of Health and Rehabilitation Research 5, 1–7 (2025).
- C. Giavatto, J. Mourani, C. Fitzpatrick, B. Hardin, A. Skrtic, A. Evans, E. Sredzinski, S. Trieu, A. I. Setter, L. Kobiska, A. I. Lopez-Medina, Biosimilar perceptions among autoimmune prescribers and pharmacists in health system specialty pharmacy. JMCP 30, 175–182 (2024).
- H. Najeeb, F. Yasmin, S. Surani, Emerging role of biosimilars in the clinical care of inflammatory bowel disease patients. World J Clin Cases 10, 4327–4333 (2022).
- D. C. Rosembert, M. J. Twigg, D. J. Wright, Patient’s and consultant’s views and perceptions on switching from an originator biologic to biosimilar medication: A qualitative study. Pharmacy 12, 65 (2024).
- L. Barbier, H. C. Ebbers, P. Declerck, S. Simoens, A. G. Vulto, I. Huys, The Efficacy, Safety, and Immunogenicity of Switching Between Reference Biopharmaceuticals and Biosimilars: A Systematic Review. Clin Pharmacol Ther 108, 734–755 (2020).
- B. G. Feagan, M. Marabani, J. J. Wu, F. Faccin, C. Spronk, G. Castañeda-Hernández, The Challenges of Switching Therapies in an Evolving Multiple Biosimilars Landscape: A Narrative Review of Current Evidence. Adv Ther 37, 4491–4518 (2020).

