- In collaboration with Reuters, Truveta Research explored trends in FDA-approved anti-obesity GLP-1 RA prescriptions among adolescents.
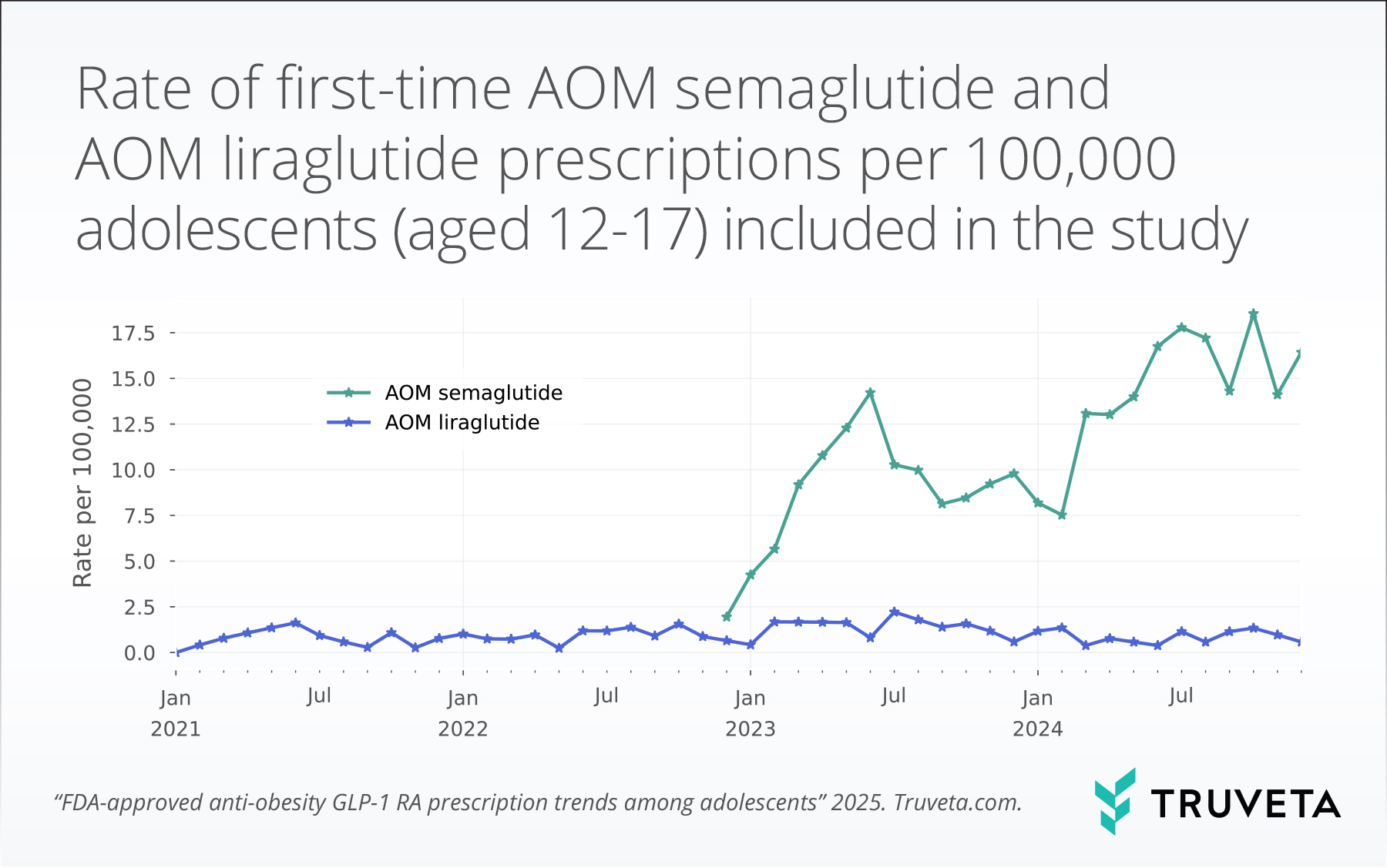
- Among the 1,265,399 adolescents (aged 12-17 years) in this study, the average rate of first-time AOM semaglutide prescriptions was 14.8 prescriptions per 100,000 adolescents in 2024. This was 1.5-fold higher than the rate in 2023, which was 9.9 prescriptions per 100,000 adolescents.
- The 2024 average first-time AOM semaglutide prescription rate was 17.7-fold higher than the first-time AOM liraglutide prescription rate.
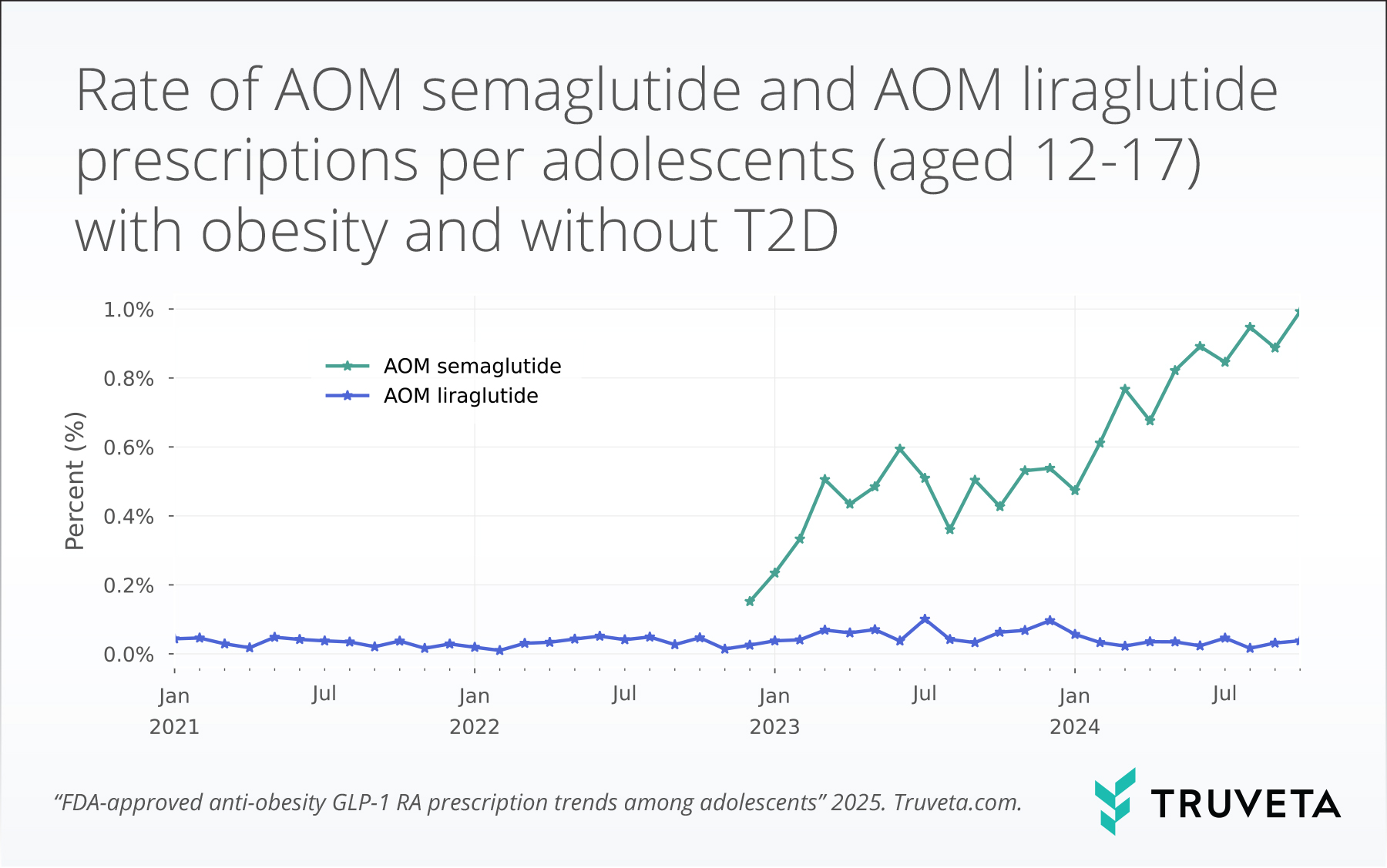
- Among the 270,170 adolescent patients with obesity, the average rate of AOM semaglutide prescriptions for adolescents with obesity within 100 days of an encounter was 0.9%. This was an increase of 88.2% or 1.9-fold higher than the rate in 2023, which was 0.5%.
- The 2024 average AOM semaglutide prescription rate was 23.7-fold higher than the AOM liraglutide prescription rate.
The use of anti-obesity medications (AOMs) in adults have both surged and been widely studied. There is growing evidence supporting the effectiveness of GLP-1 receptor agonists (GLP-1 RAs) for weight management (1–3), including some of our own Truveta research.
However, much less is known about prescribing patterns in adolescents, despite the increasing prevalence of obesity in this population.
The CDC cites that one in five children has obesity (4). Although AOM liraglutide (Saxenda) has been approved for treatment of obesity in adolescents since late 2020 (5), the approval of AOM semaglutide (Wegovy) in December 2022 for treatment of obesity for adolescents prompted new excitement (6). Semaglutide is part of the new generation of GLP-1 RA medications, which have greater weight loss efficacy than liraglutide (7).
Understanding how these medications are being used in adolescent populations is essential to inform clinical practice and assess real-world uptake. You can view the entire study directly in Truveta Studio.
Methods
Using a subset of Truveta Data, we found a population of adolescent patients (aged 12-17 years old) who had a recorded body mass index (BMI) on the same day as an outpatient visit between January 1, 2021 and March 31, 2025.
First-time AOM prescriptions for adolescents
Each month we calculated the rate of first-time AOM liraglutide or AOM semaglutide prescriptions per patients aged 12-17 years old with a BMI measurement within the year prior. In addition, we included semaglutide prescriptions where the brand was unknown, if the patient had prior evidence of obesity and no prior evidence of type 2 diabetes (T2D). This approach ensured that we captured all potential obesity-related prescriptions, while minimizing the risk of misclassifying prescriptions intended for other indications.
An example patient journey is below. The patient had their BMI measured in February 2022 and January 2023, then-received a first-time AOM GLP-1 RA prescription in July 2023. This patient was included in the study from February 2022 to July 2023 and counted as having a first-time GLP-1 RA prescription in July 2023.

This method is important for identifying first-time obesity-related prescriptions for all adolescents, ensuring that we track new treatment initiations while reducing the risk of including on-going prescriptions.
AOM prescriptions for adolescents with obesity
We classified patients with obesity as those with a BMI at or greater than the 95th percentile based on age and sex by the CDC guidelines (8). We excluded patients who had any evidence of T2D.
We calculated the monthly percentage of patients who met our inclusion criteria and who received a prescription for AOM liraglutide or AOM semaglutide within the 10 days prior or 100 days after the BMI event.
This metric looked at all prescriptions (not just first-time prescriptions, as we did with the overall population). Therefore, it is important to note a patient could be counted in multiple months if they had multiple BMI events within different months. Although Truveta Data is updated daily, we only used data through December 2024 as the last month of this analysis to allow for the 100-day prescription window.
By considering all prescriptions within a defined 100-day window around BMI events, this approach offers a broader view of prescribing trends among adolescents with obesity, allowing us to assess ongoing treatment patterns rather than just initial prescriptions.
Results
First-time AOM prescriptions for adolescents
We found 1,265,399 patients who met our inclusion criteria. 0.2% of the population received AOM liraglutide or AOM semaglutide during the study period. The majority of the population received only AOM semaglutide (88.6%) and a small percentage received prescriptions for both AOM semaglutide and AOM liraglutide (6.3%).
Over the study period, prescriptions for AOM liraglutide remained relatively stable and low, averaging 0.9 +/- 0.6 prescriptions per 100,000 adolescents. Following the approval of AOM semaglutide in December 2022, the rates of prescribing rose substantially. The average rate of first-time AOM semaglutide prescriptions per adolescents meeting inclusion criteria was 14.8 prescriptions per 100,000 adolescents in 2024. This was 1.5-fold higher than the rate in 2023, which was 9.9 prescriptions per 100,000 adolescents.
The 2024 average first-time AOM semaglutide prescription rate was 17.7-fold higher than the first-time AOM liraglutide prescription rate.
AOM prescriptions for adolescents with obesity
We found 270,170 adolescent patients who met our criteria of having obesity and no T2D.
0.5% of the population with obesity received AOM liraglutide or AOM semaglutide during the study period. Most patients received AOM semaglutide (94.1%).
Over the study period, prescriptions for AOM liraglutide remained relatively stable and low, with an average rate of 0.04 ± 0.02%. Following the approval of AOM semaglutide in December 2022, the rates of prescribing rose substantially. The 2024 average rate of AOM semaglutide prescriptions for adolescents with obesity within 100 days of an encounter was 0.9%. This was an increase 88.2% or 1.9-fold higher than the rate in 2023, which was 0.5%.
The 2024 average AOM semaglutide prescription rate amongst adolescents with obesity was 23.7-fold higher than the 2024 average AOM liraglutide prescription rate.
Discussion
Our analysis of Truveta Data reveals notable trends in the prescribing patterns of AOM semaglutide compared to AOM liraglutide, among adolescents aged 12 to 17 years between January 2021 and March 2025. These findings provide valuable insights into the evolving landscape of obesity management in this population.
Increasing prescriptions of GLP-1 RAs in adolescents
These data indicate a substantial rise in AOM semaglutide, following its approval for adolescent obesity management in late 2022 (6). This trend aligns with national dispensing patterns, where a study reported a nearly 600% increase in GLP-1 RA prescription fills among adolescents and young adults from 2020 to 2023 (9) and our own research, which has shown that GLP-1 RAs now make up over 5% of all prescriptions prescribed. The increase in semaglutide prescriptions may be attributed to its demonstrated efficacy in promoting weight loss.
Each analysis in our study provides unique insights into the prescribing patterns of AOM GLP-1 RAs among adolescents. The overall population analysis captures broad prescribing trends, offering a comprehensive view of how often these medications are initiated among all adolescents. This approach helps assess real-world use and adoption following regulatory approvals. In contrast, the obesity-specific analysis focuses on adolescents who meet the CDC definition of obesity and have no prior evidence of T2D. This subgroup allows for a more targeted evaluation of AOM GLP-1 RA use in the population consistent with the medication label and looks at the total number of prescriptions. By comparing both analyses, we can distinguish general prescribing trends from those specific to adolescents with obesity, helping to contextualize the extent to which these medications are used in alignment with clinical guidelines.
Implications for clinical practice
The increasing prescription rates of AOM semaglutide among adolescent may reflect a shift towards pharmacological interventions in managing pediatric obesity. This trend underscores the importance of monitoring how recent robust clinical guidelines are being followed to ensure appropriate patient selection, dosing, and monitoring. Healthcare providers should weigh the benefits of significant weight reduction against potential risks, emphasizing a comprehensive approach that includes lifestyle modifications and psychosocial support.
While this study did not investigate effectiveness for either medication, its safety profile warrants careful consideration. Common adverse reactions observed in adolescents include headache, abdominal pain, nausea, vomiting, and diarrhea, mirroring those reported in adult populations (10, 11). Additionally, concerns have been raised about the potential long-term effects of GLP-1 RAs on mental health. While recent studies suggest that semaglutide may offer mental health benefits—such as a study involving teenagers with obesity reporting a 33% reduction in suicidal ideation or attempts over 12 months (12)—evidence on suicidality risk remains mixed. Some reports have suggested a potential increase in suicidality risk associated with semaglutide, though the preponderance of current evidence does not indicate a heightened risk (13, 14). Potential protective effects may arise from multiple mechanisms, including the complex interplay between weight stigmatization, psychological distress, and improvements in overall health and self-perception among adolescents (15, 16). Nonetheless, the long-term consequences of GLP-1 RA use in adolescents remain under-researched, highlighting the need for future studies on safety and effectiveness in adolescent populations.
Limitations
In this study, we may over-classify patients with semaglutide; however, the approach we used attempted to ensure we were not missing people with prescriptions for AOM semaglutide. Future research is needed to study the use of obesity treatments other than AOM liraglutide and AOM semaglutide, including lifestyle interventions and use of other GLP-1 and non-GLP-1 anti-obesity medications.
These are preliminary research findings and not peer reviewed. Data are constantly changing and updating. These findings are consistent with data accessed on April 16, 2025. You can view the entire study directly in Truveta Studio.
Citations
- M. Shah, A. Vella, Effects of GLP-1 on appetite and weight. Rev Endocr Metab Disord 15, 181–187 (2014).
- A. M. Jastreboff, L. J. Aronne, N. N. Ahmad, S. Wharton, L. Connery, B. Alves, A. Kiyosue, S. Zhang, B. Liu, M. C. Bunck, A. Stefanski, Tirzepatide Once Weekly for the Treatment of Obesity. N Engl J Med 387, 205–216 (2022).
- W. T. Garvey, R. L. Batterham, M. Bhatta, S. Buscemi, L. N. Christensen, J. P. Frias, E. Jódar, K. Kandler, G. Rigas, T. A. Wadden, S. Wharton, the STEP 5 Study Group, Two-year effects of semaglutide in adults with overweight or obesity: the STEP 5 trial. Nat Med 28, 2083–2091 (2022).
- Denters for Disease Control and Prevention, Childhood Obesity Facts, Obesity (2024). https://www.cdc.gov/obesity/childhood-obesity-facts/childhood-obesity-facts.html.
- U.S. Food & Drug Administration, “FDA approves weight management drug for patients aged 12 and older” (2021); https://www.fda.gov/drugs/news-events-human-drugs/fda-approves-weight-management-drug-patients-aged-12-and-older.
- Novo Nordisk, FDA approves once-weekly Wegovy® injection for the treatment of obesity in teens aged 12 years and older (2022). https://www.novonordisk-us.com/media/news-archive/news-details.html?id=151389.
- D. M. Rubino, F. L. Greenway, U. Khalid, P. M. O’Neil, J. Rosenstock, R. Sørrig, T. A. Wadden, A. Wizert, W. T. Garvey, STEP 8 Investigators, C. Arauz-Pacheco, K. Cannon, H. J. Downey, D. Fitz-Patrick, J. Geohas, G. Gerety, J. Gilbert, P. Hollander, E. Klein, K. Laufer, P. O’Donnell, P. Rosenblit, P. Toth, Effect of Weekly Subcutaneous Semaglutide vs Daily Liraglutide on Body Weight in Adults With Overweight or Obesity Without Diabetes: The STEP 8 Randomized Clinical Trial. JAMA 327, 138 (2022).
- Centers for Disease Control and Prevention, CDC Extended BMI-for-age Growth Charts, National Center for Health Statistics (2024). https://www.cdc.gov/growthcharts/extended-bmi.htm.
- J. M. Lee, M. Sharifi, L. Oshman, D. H. Griauzde, K.-P. Chua, Dispensing of Glucagon-Like Peptide-1 Receptor Agonists to Adolescents and Young Adults, 2020-2023. JAMA 331, 2041 (2024).
- P. M. Ryan, S. Seltzer, N. E. Hayward, D. A. Rodriguez, R. T. Sless, C. P. Hawkes, Safety and Efficacy of Glucagon-Like Peptide-1 Receptor Agonists in Children and Adolescents with Obesity: A Meta-Analysis. The Journal of Pediatrics 236, 137-147.e13 (2021).
- L. B. T. Yugar, L. G. Sedenho-Prado, I. M. C. Da Silva Ferreira, C. A. M. Silva, A. C. Sposito, C. Cercato, The efficacy and safety of GLP-1 receptor agonists in youth with type 2 diabetes: a meta-analysis. Diabetol Metab Syndr 16, 92 (2024).
- L. Kerem, J. Stokar, Risk of Suicidal Ideation or Attempts in Adolescents With Obesity Treated With GLP1 Receptor Agonists. JAMA Pediatr 178, 1307 (2024).
- U.S. Food & Drug Administration, Update on FDA’s ongoing evaluation of reports of suicidal thoughts or actions in patients taking a certain type of medicines approved for type 2 diabetes and obesity (2024). https://www.fda.gov/drugs/drug-safety-and-availability/update-fdas-ongoing-evaluation-reports-suicidal-thoughts-or-actions-patients-taking-certain-type.
- W. Wang, N. D. Volkow, N. A. Berger, P. B. Davis, D. C. Kaelber, R. Xu, Association of semaglutide with risk of suicidal ideation in a real-world cohort. Nat Med 30, 168–176 (2024).
- J. L. Warnick, K. E. Darling, C. E. West, L. Jones, E. Jelalian, Weight Stigma and Mental Health in Youth: A Systematic Review and Meta-Analysis. Journal of Pediatric Psychology 47, 237–255 (2022).
- P. Baiden, C. Cañizares, C. A. LaBrenz, C. M. Sellers, Y. Li, R. M. Glikpo, K. Sarkodie, Effects of objective and perceived weight on suicidal ideation among adolescents: Findings from the 2015–2021 national Youth Risk Behavior Survey. Psychiatry Research 345, 116380 (2025).