Today Truveta announced new comparative effectiveness research exploring weight loss among patients taking semaglutide (Ozempic) and tirzepatide (Mounjaro).
Glucagon-like peptide 1 receptor agonist-based (GLP-1 RA) medications, including semaglutide and the dual GLP-1 RA/gastric inhibitory polypeptide (GIP) medication tirzepatide, are used to treat type 2 diabetes or obesity. In this study, Truveta Research explored weight loss trends for patients taking semaglutide and tirzepatide approved by the FDA for treating type 2 diabetes (Ozempic and Mounjaro, respectively).
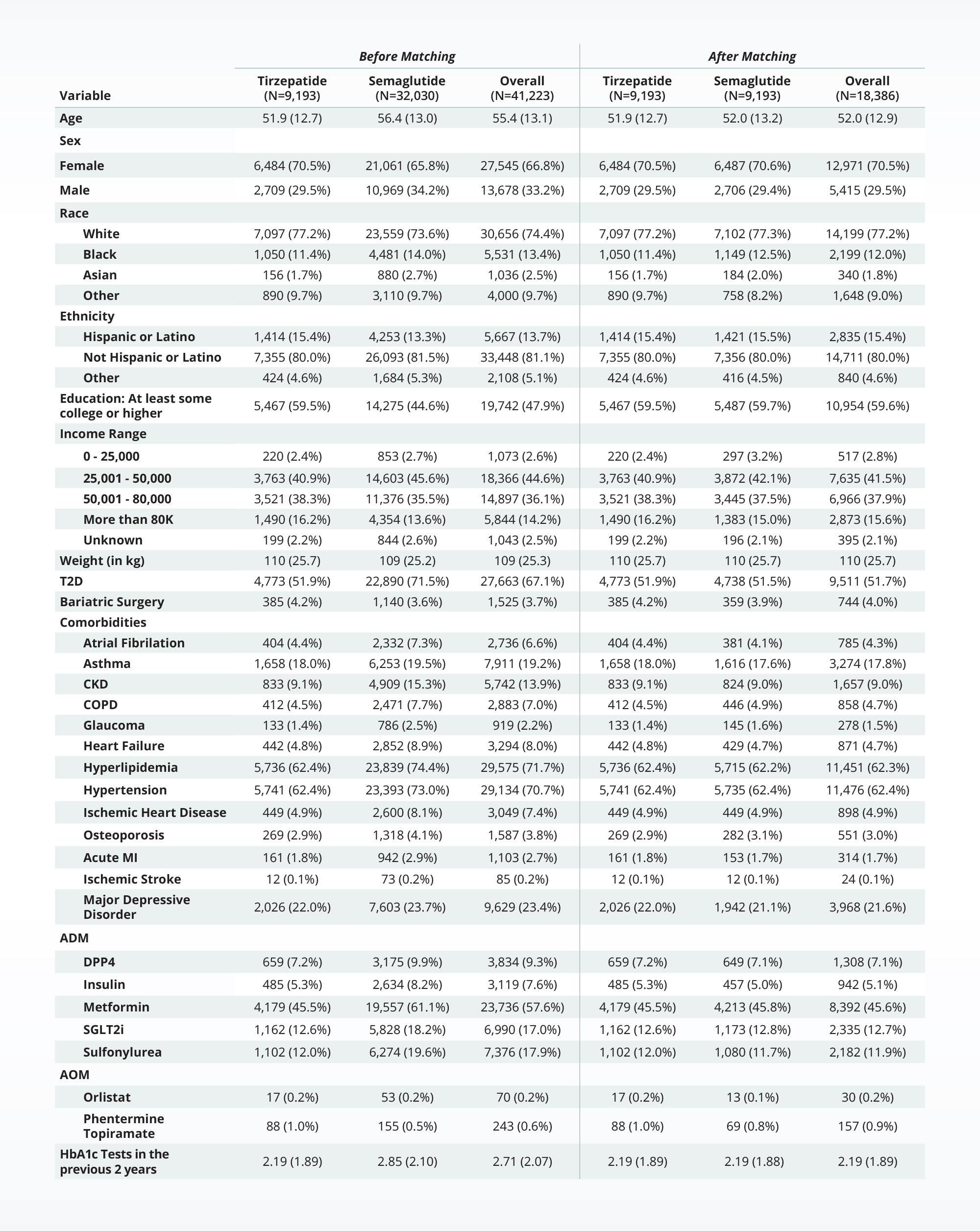
Unadjusted populations for semaglutide and tirzepatide differ
Using Truveta Data, Truveta Research began with a patient population of over 40,000 patients with overweight or obesity initiating either semaglutide or tirzepatide between May 2022 and September 2023.
The study found that in the initial unadjusted population, those taking tirzepatide and semaglutide differed (the “Before Matching” population in the table below).
While both populations are primarily white and female, those taking tirzepatide are more likely to be younger, have at least some college education, have fewer co-morbidities, and not have type 2 diabetes.
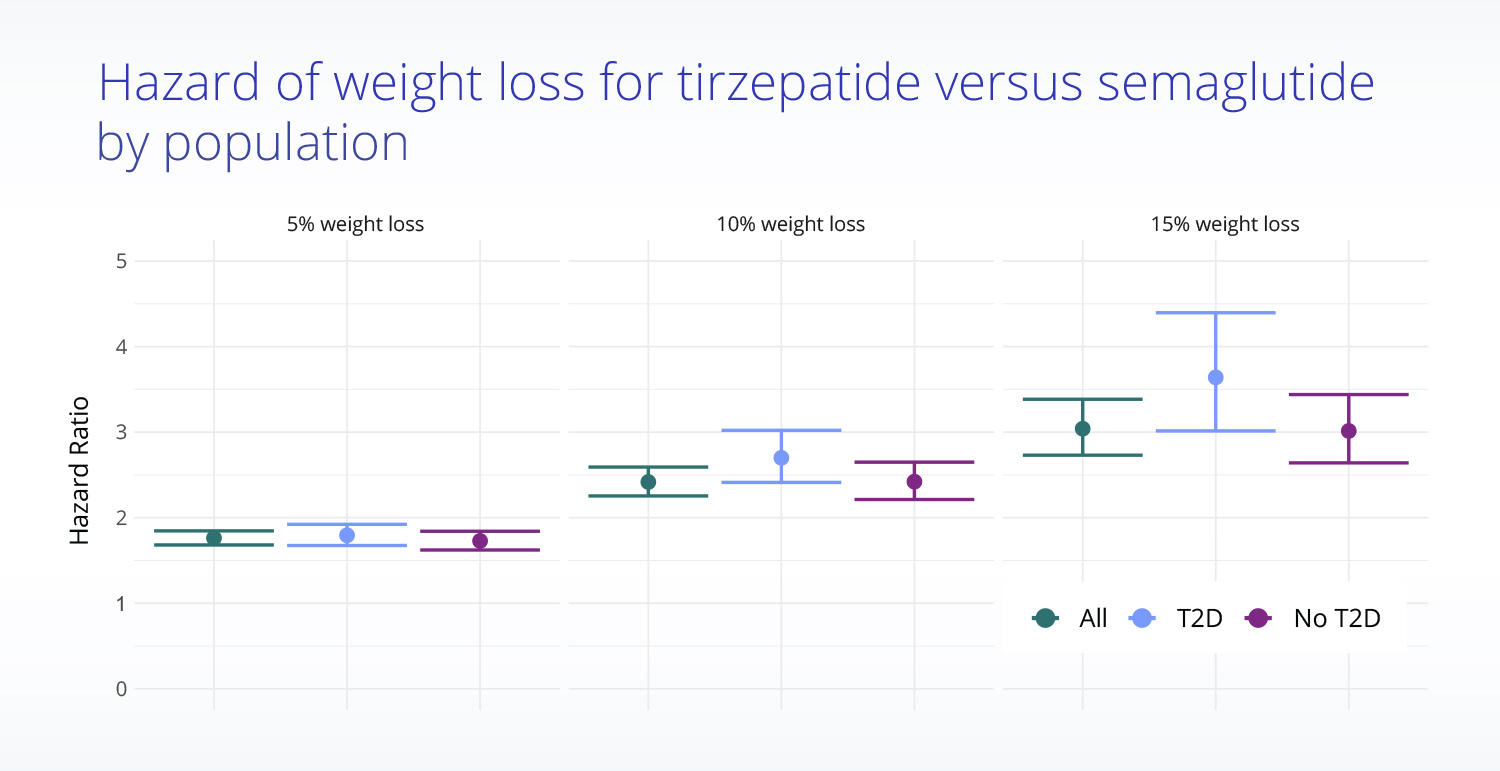
Adjusted population analysis finds tirzepatide more effective than semaglutide for weight loss
Truveta Research then used multiple rigorous methods (including propensity score matching) to achieve a well-balanced analytic cohort of 18,000 patients to appropriately evaluate the effectiveness of semaglutide compared with tirzepatide for weight loss.
The analysis found that patients taking tirzepatide were significantly more likely to achieve weight loss:
- Those taking tirzepatide were 1.8 times more likely than those taking semaglutide to achieve 5% weight loss,
- 2.4 times more likely than those taking semaglutide to achieve 10% weight loss,
- And three times more likely than those taking semaglutide to achieve 15% weight loss.
Truveta Research also found that patients taking tirzepatide experienced significantly larger reductions in body weight at specified timepoints:
- At 3 months, the mean percentage change in body weight was -5.9% for those taking tirzepatide versus -3.6% for those taking semaglutide.
- At six months, the mean percentage change in body weight was -10.1% for those taking tirzepatide versus -5.9% for those taking semaglutide.
- At one year, the mean percentage change in body weight was -15.2% for those taking tirzepatide versus -7.9% for those taking semaglutide.
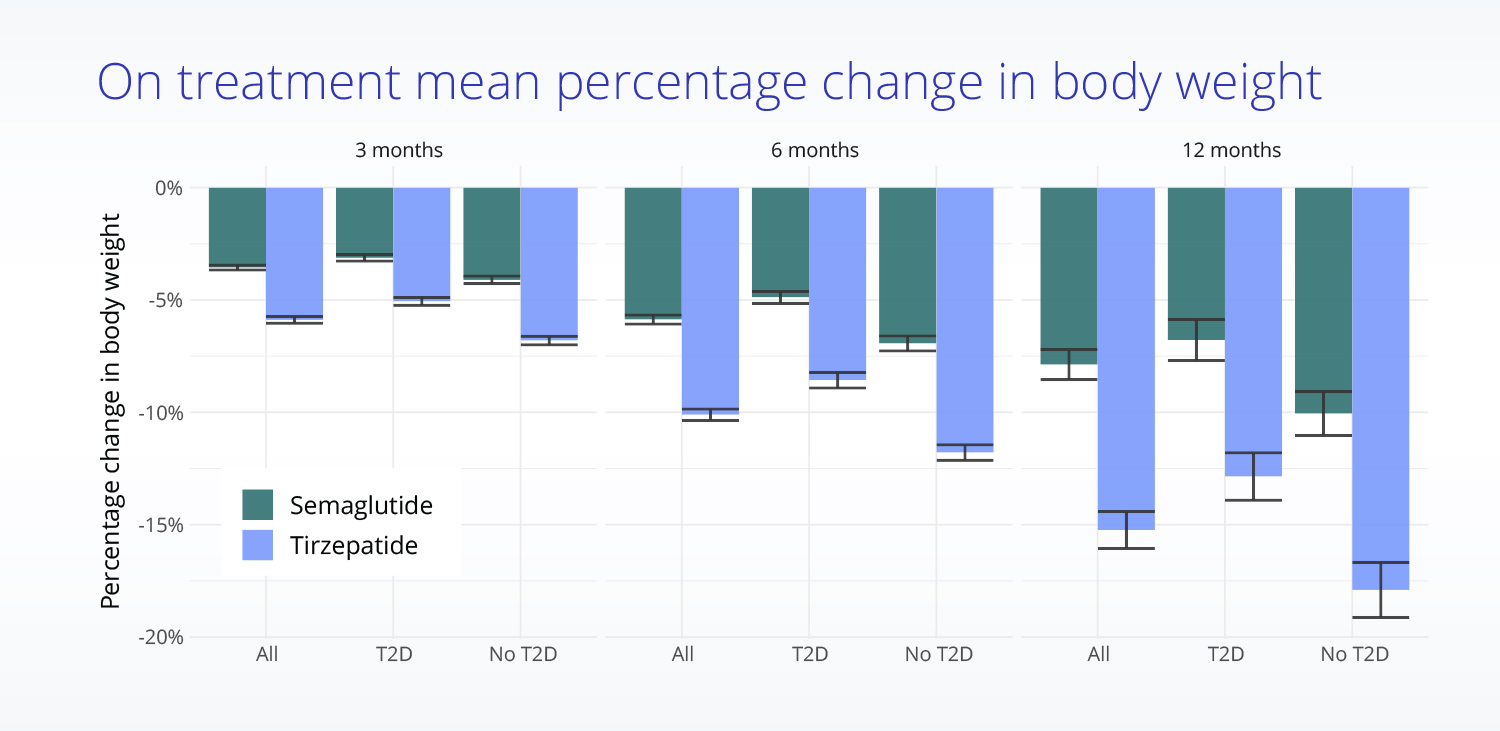
Additionally, Truveta Research found that weight loss was greater for patients without type 2 diabetes than for those with evidence of type 2 diabetes, though differences in effectiveness between tirzepatide and semaglutide were similar.
Rates of gastrointestinal adverse events were similar between those taking tirzepatide and semaglutide.
The full study is available on MedRxiv.
Perspective from authors
Nick Stucky, MD, PhD, vice president of Truveta Research, practicing infectious disease physician at Providence Health, and a co-author on the study shared the impact of having real-world data to study the efficacy of these treatments:
Tyler Gluckman, MD, MHA, FACC, FAHA, FASPC, another author on the paper and cardiologist at Providence Health, as well as the medical director at the Center for Cardiovascular Analytics, Research, and Data Science (CARDS) at the Providence Heart Institute, shares his perspective on the importance of timely data to assess the real-world effect of medications to inform patient care.


