Blog
Featured post
Regulatory-grade device evidence reimagined with new precision data
By linking unique device identifier (UDI) data with minute-level Admission–Discharge–Transfer (ADT), procedure logs, and chargemaster data, Truveta delivers the full picture of when, how, and in what context devices are used—accelerating regulatory submissions, health economics and outcomes research (HEOR), safety monitoring, and more.
Associations of nutrition counseling, insulin, and metformin therapy with post-GTT weight gain in a large pregnancy cohort
Nearly half of patients with gestational diabetes mellitus (GDM) received nutrition counseling within 45 days of diagnosis, while smaller proportions initiated insulin (15%) or...
Real-world patterns of glucose tolerance testing and gestational diabetes in pregnancy
Nearly one-quarter of patients exceeded the 140 mg/dL threshold on the 1-hour glucose tolerance test. However, about 1 in 10 patients with elevated values did not receive...
Truveta experts: Using real-world data to track seasonal Legionella trends across the United States
In this Truveta experts spotlight, Amy Wu, Director, Partner Research and Success, analyzed recent seasonal and regional patterns in Legionella cases across the US using Truveta...
Advancing clinical information extraction with LLM-Augmenter
Extracting structured information from clinical notes—such as identifying medical conditions, medications, and their relationships—is a cornerstone of modern healthcare research....
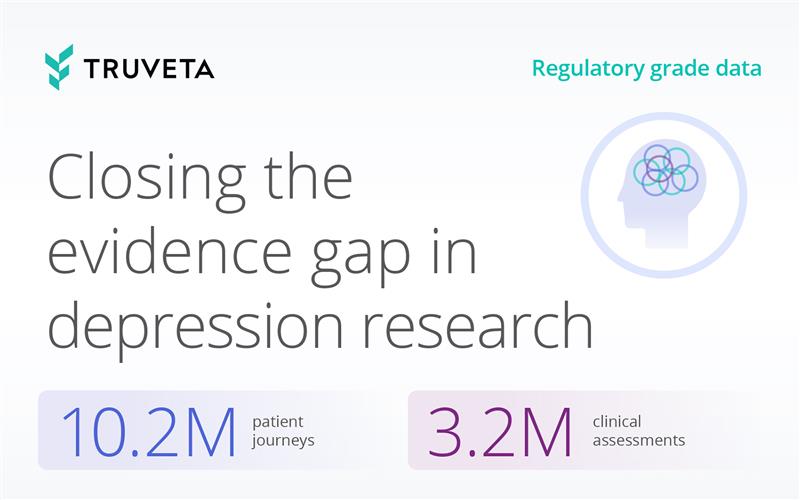
Closing the evidence gap in depression research
Major depressive disorder (MDD) is one of the most urgent and costly public health challenges today. More than 18% of US adults live with depression, and rates are even higher...
CDC study highlights need for code to track new STI prevention tool
Doxycycline postexposure prophylaxis (doxy PEP) is now recommended by the CDC to help prevent bacterial STIs in certain high-risk groups, a prevention strategy that highlights...
Transparency is the new standard: FDA’s view on regulatory-grade RWE
The US Food and Drug Administration (FDA) recently published a comprehensive overview of how real-world evidence (RWE) has contributed to regulatory decision-making since 2011....
Exploring gestational weight gain and newborn birth weight
Mothers who gained more weight during pregnancy had heavier infants and mothers who lost weight during pregnancy had lighter infants. Mothers who gained less weight (particularly...
Exploring gestational weight gain and loss
Across their pregnancies, mothers had an average of 12 weight measurements, allowing us to construct a longitudinal ‘journey’ of their weight change. Mothers gained an average of...