Blog
Featured post
Regulatory-grade device evidence reimagined with new precision data
By linking unique device identifier (UDI) data with minute-level Admission–Discharge–Transfer (ADT), procedure logs, and chargemaster data, Truveta delivers the full picture of when, how, and in what context devices are used—accelerating regulatory submissions, health economics and outcomes research (HEOR), safety monitoring, and more.
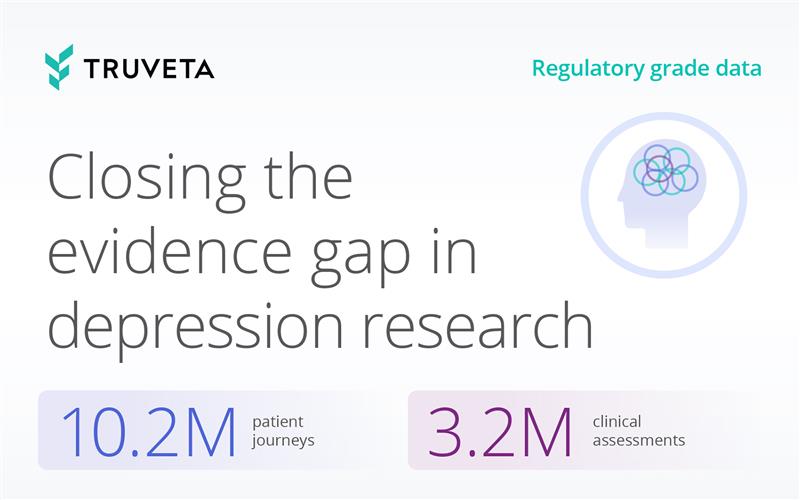
Closing the evidence gap in depression research
Major depressive disorder (MDD) is one of the most urgent and costly public health challenges today. More than 18% of US adults live with depression, and rates are even higher...
CDC study highlights need for code to track new STI prevention tool
Doxycycline postexposure prophylaxis (doxy PEP) is now recommended by the CDC to help prevent bacterial STIs in certain high-risk groups, a prevention strategy that highlights...
Transparency is the new standard: FDA’s view on regulatory-grade RWE
The US Food and Drug Administration (FDA) recently published a comprehensive overview of how real-world evidence (RWE) has contributed to regulatory decision-making since 2011....
Exploring gestational weight gain and newborn birth weight
Mothers who gained more weight during pregnancy had heavier infants and mothers who lost weight during pregnancy had lighter infants. Mothers who gained less weight (particularly...
Exploring gestational weight gain and loss
Across their pregnancies, mothers had an average of 12 weight measurements, allowing us to construct a longitudinal ‘journey’ of their weight change. Mothers gained an average of...
Boston Scientific finds reduced infection risk with pressure monitoring ureteroscope
Infections secondary to elevated intrarenal pressure and intrarenal backflow remain one of the most concerning complications following ureteroscopy (URS), a common procedure to...
Truveta experts: Adoption of MRSA testing for guiding treatment decisions
Methicillin-resistant Staphylococcus aureus (MRSA) is one of the most serious infections, often leading healthcare providers to start powerful, broad-spectrum antibiotics like...
Truveta adds new precision device data
Medical device manufacturers and regulators face a persistent challenge: obtaining a complete, clinically rich view of how devices are used, how safe they are, and how they...
Celebrating 5 years of Saving Lives with Data
Today marks the 5 year anniversary of Truveta. I personally am so humbled by where we are today. As I reflect back to where we were in 2020, the world was a very different place....