Truveta Live Link
Turn your data into more complete real-world evidence and prospective research
Thank you for your interest!
Link to daily updated data for ongoing insights
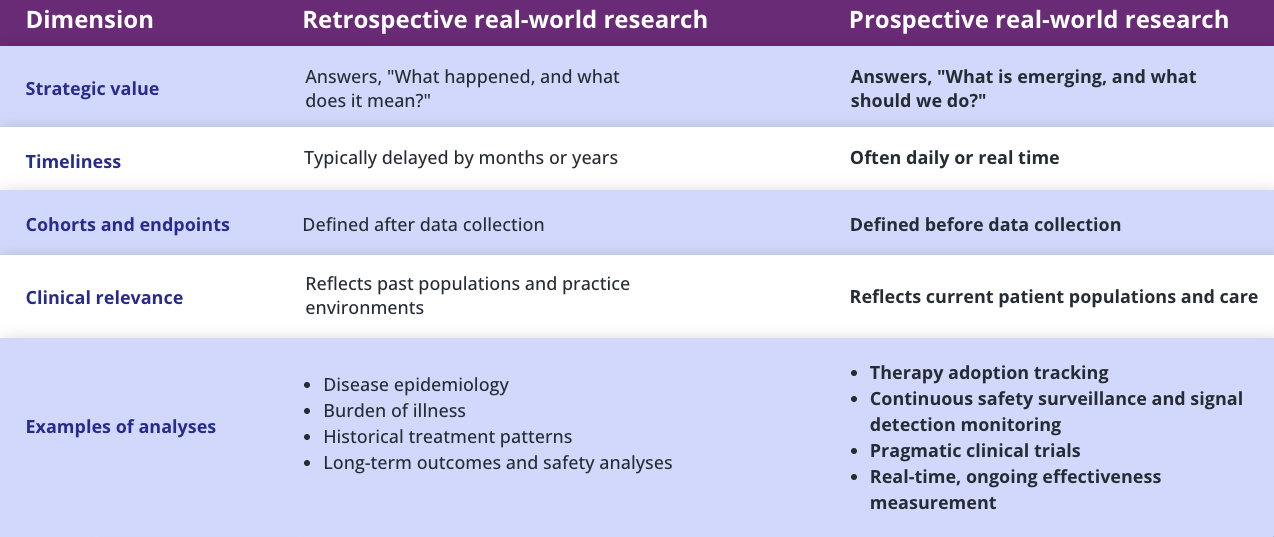
Truveta Live Link transforms life science organizations’ proprietary data by linking to daily updated EHR data from Truveta, enabling them to generate more complete, regulatory-grade evidence and prospective research that can replace costly trials and registries.
By uniting continuously updated data, expert determination, and integrated storage, Live Link empowers organizations to modernize their research strategies—accelerating discovery, reducing cost, and advancing market leadership.
Complete, longitudinal evidence from your linked data
Truveta Live Link connects proprietary data with Truveta’s complete real-world clinical data—spanning diverse patient populations and with detailed context across diagnoses, treatments, labs, and outcomes—to deliver ongoing evidence across the full patient journey.
By linking to Truveta Data, customers can confidently generate clinically rich, transparent, and audit-ready evidence in real time that exceeds regulatory standards.

Clinical trials
- Prospectively capture supplemental data for real-world control arms and pragmatic trials
- Continuously follow enrolled trial cohorts as new clinical outcomes emerge, without re-linking
HEOR
- Instantly update clinical and economic outcome evidence for cohorts derived from your data
- Bring daily refreshed insights on your therapies to HEOR, commercial, and market access teams

Safety
- Track real-time effectiveness and safety signals from your proprietary cohorts
- Longitudinally monitor linked cohorts after known safety events to track outcomes and utilization

Unmet need
- Continuously observe how therapies are adopted and used in practice as linked data refreshes
- Instantly quantify gaps between eligibility, treatment, and outcomes for your cohorts of interest

R&D
- Combine evolving EHR and proprietary data to train and validate AI models over time
- Enable ongoing model improvement as new events and outcomes are added to the linked dataset
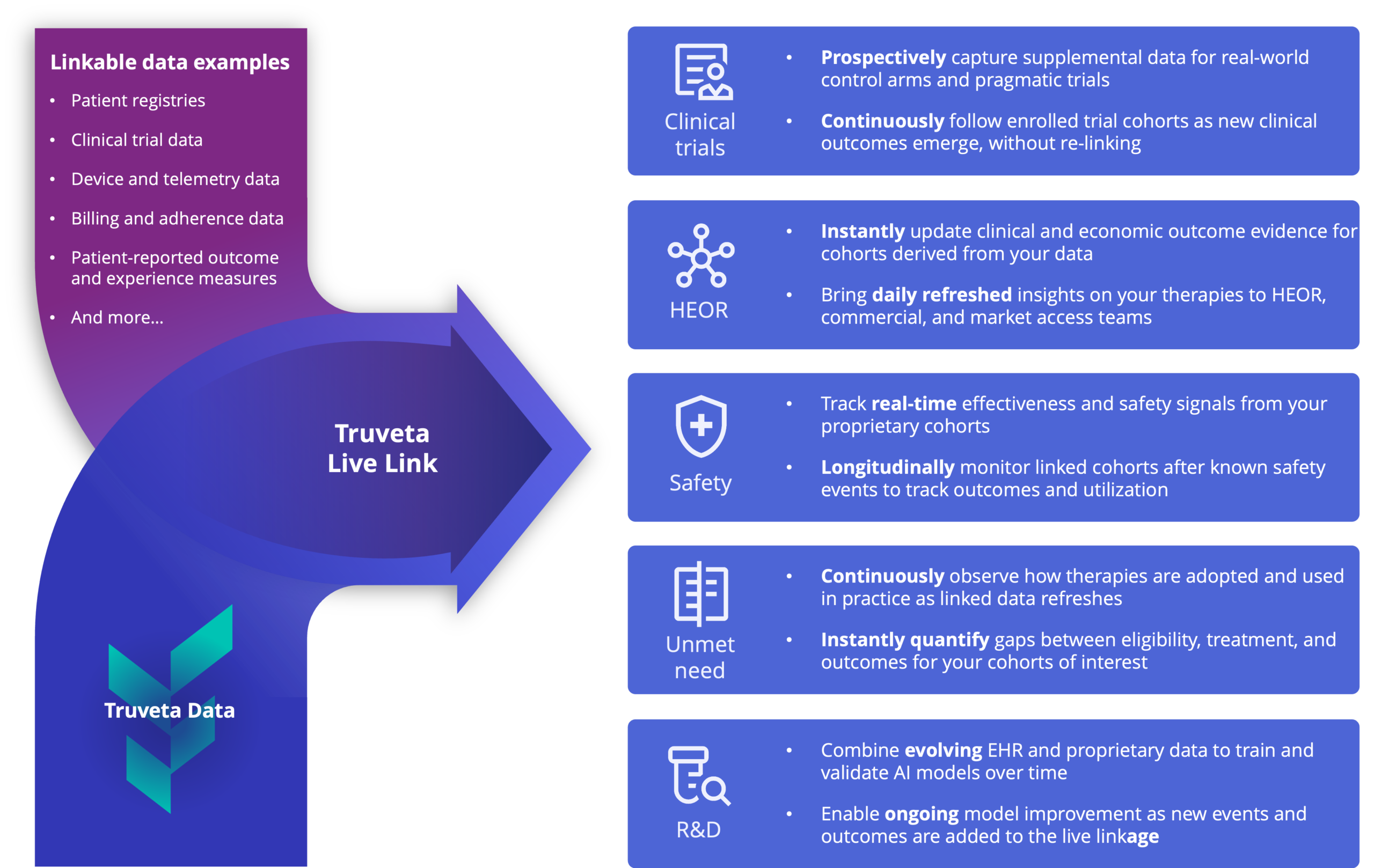
Prospective research powered by continuously updated EHR data
Move beyond static analyses and track cohorts over time for prospective evidence and research. With real-time, ongoing visibility into therapy adoption, performance, and safety trends, bridge evidence gaps and detect emerging insights and signals faster and at a lower cost than traditional trials or registries.
Dimension
Retrospective real-world research
Prospective real-world research
Strategic value
Answers, “What happened, and what does it mean?”
Answers, “What is emerging, and what should we do?”
Timeliness
Typically delayed by months or years
Often daily or real time
Cohorts and endpoints
Defined after data collection
Defined before data collection
Clinical relevance
Reflects past populations and practice environments
Reflects current patient populations and care
Examples of analyses
- Disease epidemiology
- Burden of illness
- Historical treatment patterns
- Long-term outcomes and safety analyses
- Therapy adoption tracking
- Continuous safety surveillance and signal detection monitoring
- Pragmatic clinical trials
- Real-time, ongoing effectiveness measurement
Streamlined, secure data and infrastructure
Truveta Live Link reduces cost and complexity through one integrated, token-agnostic solution.

Unlock audit-ready, de-identified linked data with expert determination included at no additional cost.

Consolidate storage, linking, and compliance into a single solution that reduces redundant contracts and third-party fees.

Leverage the same HIPAA- and HITRUST-certified infrastructure trusted by leading US health systems.



Case study
Truveta Live Link enables a leading medical device company to integrate proprietary continuous glucose monitor data with longitudinal EHR data from Truveta Data, unlocking novel real-world research to advance diabetes care.