What happens to cardiovascular health when patients stop taking glucagon-like peptide-1 receptor agonist (GLP-1 RA) medications? That’s the focus of new research from investigators at Baylor Scott & White Research Institute and Stony Brook University Hospital, presented at the European Society of Cardiology (ESC) Congress 2025.
Their abstract, “One-year cardiovascular outcomes before and after GLP-1 RA discontinuation: a real-world retrospective cohort study,” used Truveta Data to evaluate how early versus long-term treatment affects risk of major cardiovascular events.
GLP-1 RAs are widely prescribed for people with overweight, obesity, and type 2 diabetes, and are known to deliver cardiovascular benefits. Yet little is known about what happens when therapy is discontinued—an increasingly important question as real-world discontinuation rates remain high.
Methods
This real-world retrospective cohort study used Truveta Data, which includes de-identified electronic health record (EHR) data from more than 120 million patients across the US. Adults with a body mass index of at least 27 who initiated injectable semaglutide or tirzepatide between July 1, 2022 and December 31, 2024, were eligible. Participants had to have at least two prescriptions, a treatment duration of 90 days or longer, and a minimum of one year of follow-up. Discontinuation was defined as a gap of at least 90 days after the last prescription. Patients with less than one year of follow-up or ongoing treatment at study end were excluded.
The primary outcomes were the one-year cumulative incidence of acute coronary syndromes, coronary artery disease encounters, heart failure, stroke, and all-cause mortality, assessed both during treatment and after discontinuation. Propensity score matching was used to adjust for baseline conditions.
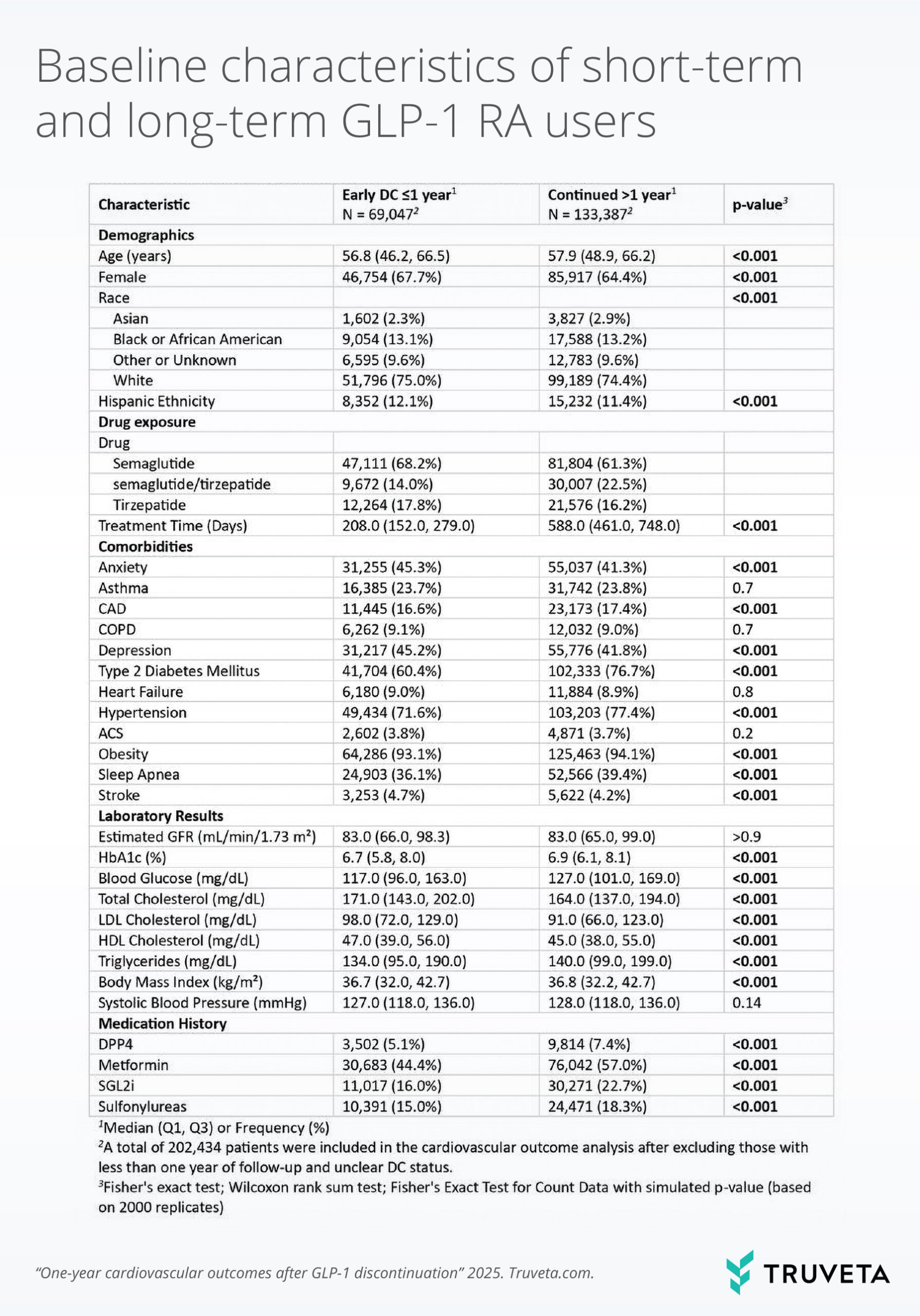
Table 1. Baseline characteristics of short-term (≤1 year) and long-term (>1 year) GLP-1 RA users.
Results
The study included 289,809 adults (median age 54.6 years, 65.2% female, 69.2% with type 2 diabetes). Discontinuation was common, with 29.6% stopping within one year and 57.3% by two years. After exclusions, the final cohort comprised 69,047 short-term users and 133,387 long-term users.
During treatment, the one-year incidence of events was 1.3% for acute coronary syndromes, 13.2% for coronary artery disease, 7.3% for heart failure, 1.9% for stroke, and 0.06% for all-cause mortality. Short-term users faced higher risks of each outcome compared to long-term users.
After discontinuation, overall event rates increased, with coronary artery disease at 17.1%, heart failure at 10.2%, and stroke at 2.6%. Elevated risks of coronary artery disease and heart failure persisted in those who discontinued early, while risks of acute coronary syndromes, stroke, and mortality were similar between groups.
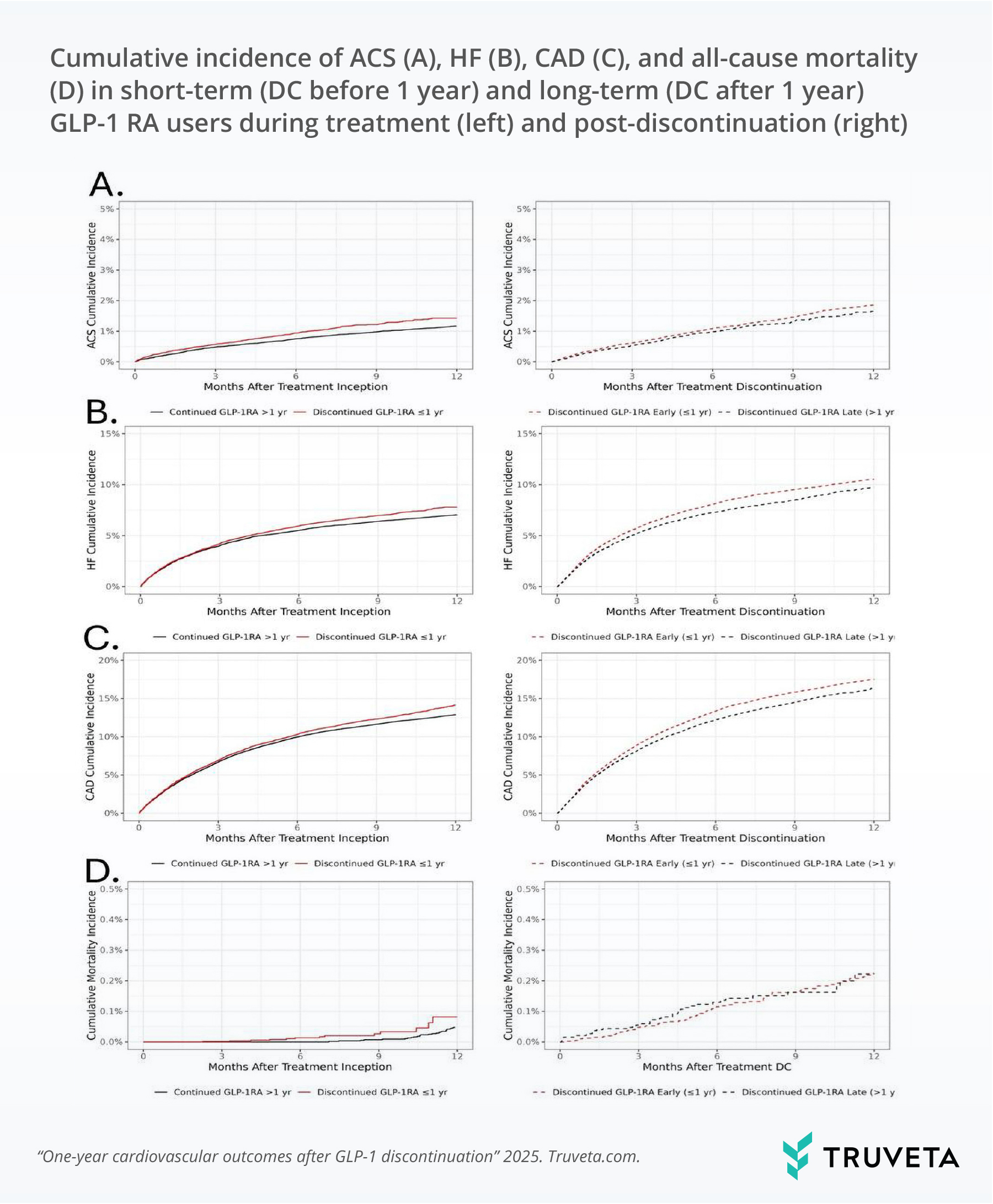
Figure 1. Cumulative incidence of ACS, HF, CAD, and all-cause mortality during treatment (left) and post-discontinuation (right), comparing short-term and long-term GLP-1 RA users.
Discussion
This study shows that early discontinuation of GLP-1 RAs is associated with higher risks of cardiovascular events during treatment. Importantly, the increased risks of coronary artery disease and heart failure persisted even after discontinuation, while the risks of acute coronary syndromes, stroke, and all-cause mortality were similar between early discontinuers and those who continued therapy. Finally, event rates increased across the board after GLP-1 RA discontinuation (regardless of treatment duration).
These findings suggest that early discontinuation may diminish the cardiovascular benefits of GLP-1 RAs, underscoring the importance of treatment persistence when evaluating real-world outcomes.
By leveraging real-world EHR data at scale, this research provides new evidence to inform both clinicians and policymakers as they weigh the role of GLP-1 RA therapy in cardiovascular care.
This research was presented at the European Society of Cardiology (ESC) Congress 2025. To learn how Truveta Data can support cardiometabolic research, contact us.
