Device evidence, reimagined
Research-ready data with unmatched precision and traceability
Thank you for your interest!
Device-level data from EHRs, claims, and chargemaster
Breadth and depth with full traceability
Traditional, standalone data sources—claims, chargemaster, and registries—were never built for lifecycle-wide evidence. They provide fragments of the picture but, alone, cannot deliver the precision, speed, or representativeness regulators and payers now expect.
Truveta’s governing health systems contribute de-identified EHR and chargemaster data directly, delivering unmatched clinical depth with full provenance back to source. Linked claims extend that insight, providing cost and utilization data to power robust economic analyses with clinical context.
130M+
patients
45M+
10+
4B+
5M+
10M+
Purpose-built for device evidence
Get the details needed to study utilization, safety, effectiveness, and cost with confidence.
Device-level granularity
Device-level granularity
Admission–Discharge–Transfer (ADT) data
Admission–Discharge–Transfer (ADT) data
Procedure log data
Procedure log data
Linked chargemaster
Linked chargemaster
Clinical notes
Clinical notes
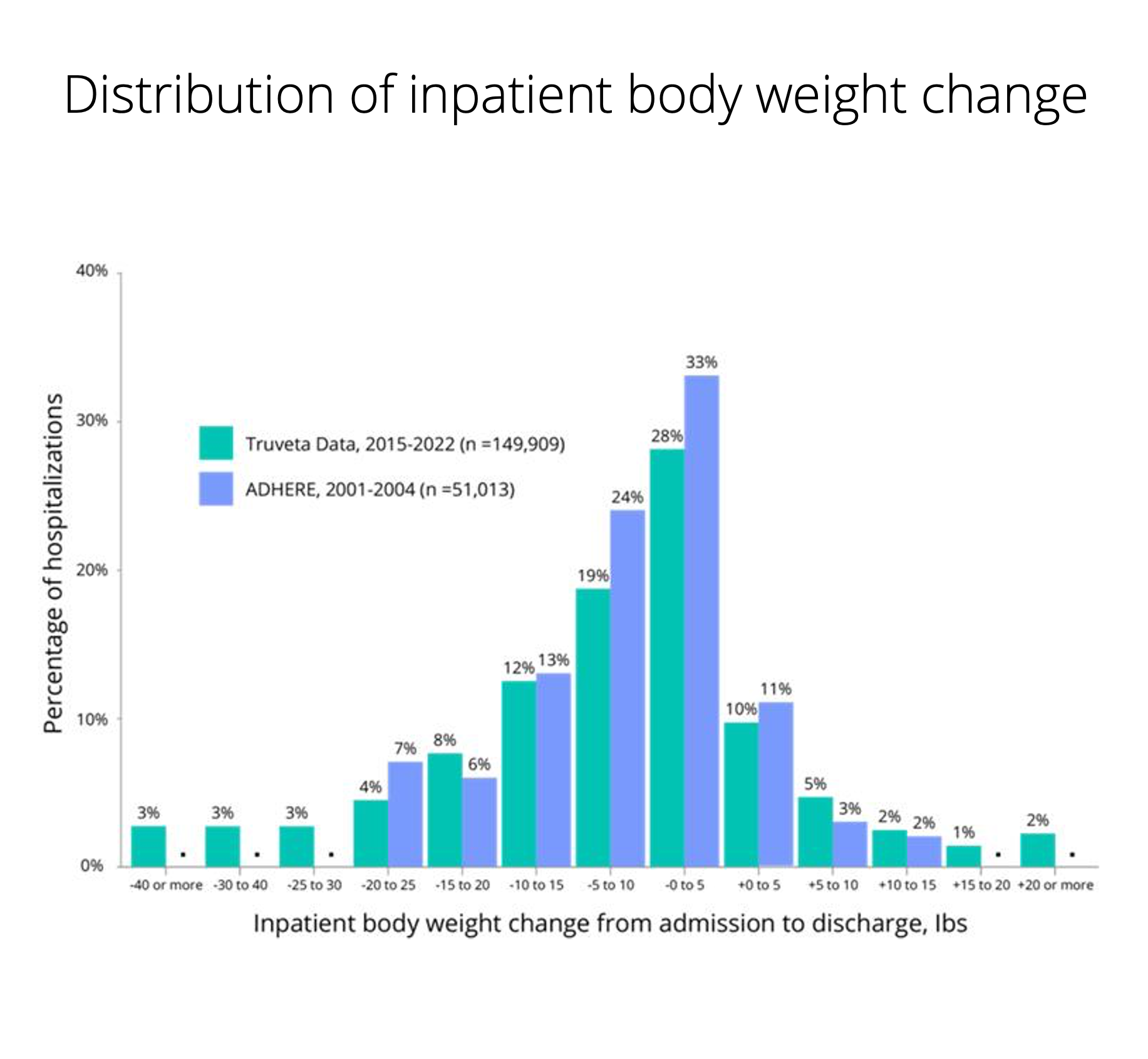
Imaging at scale
Imaging at scale
Evidence across the lifecycle, in any therapeutic area

De-risk trials with real-world feasibility modeling, real-time site- and provider-level visibility, and partnership opportunities with leading US health systems.

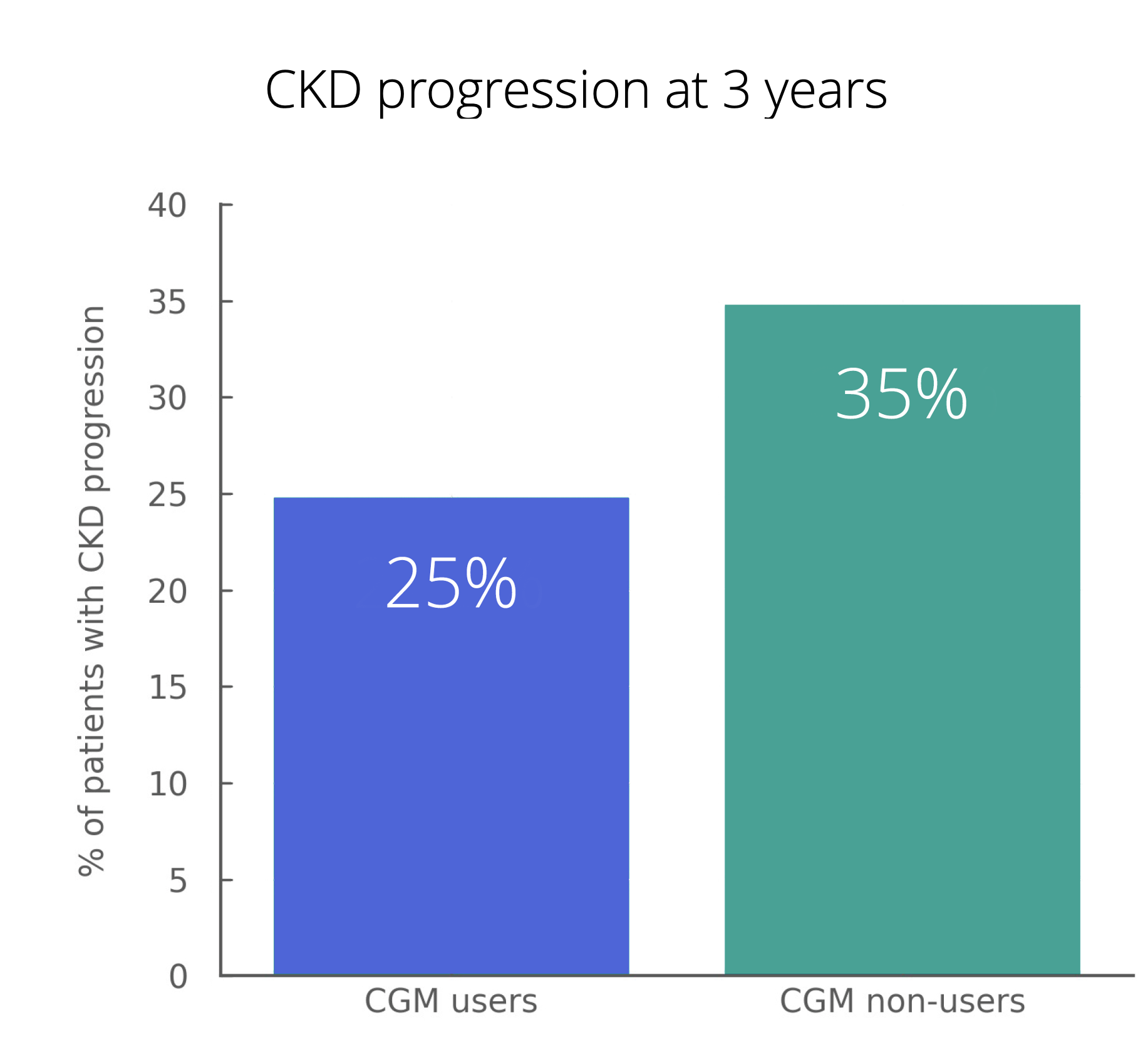
Demonstrate value faster to payers and providers by linking device use to utilization, outcomes, and costs.

Expand total addressable market by uncovering disparities in access and adoption.

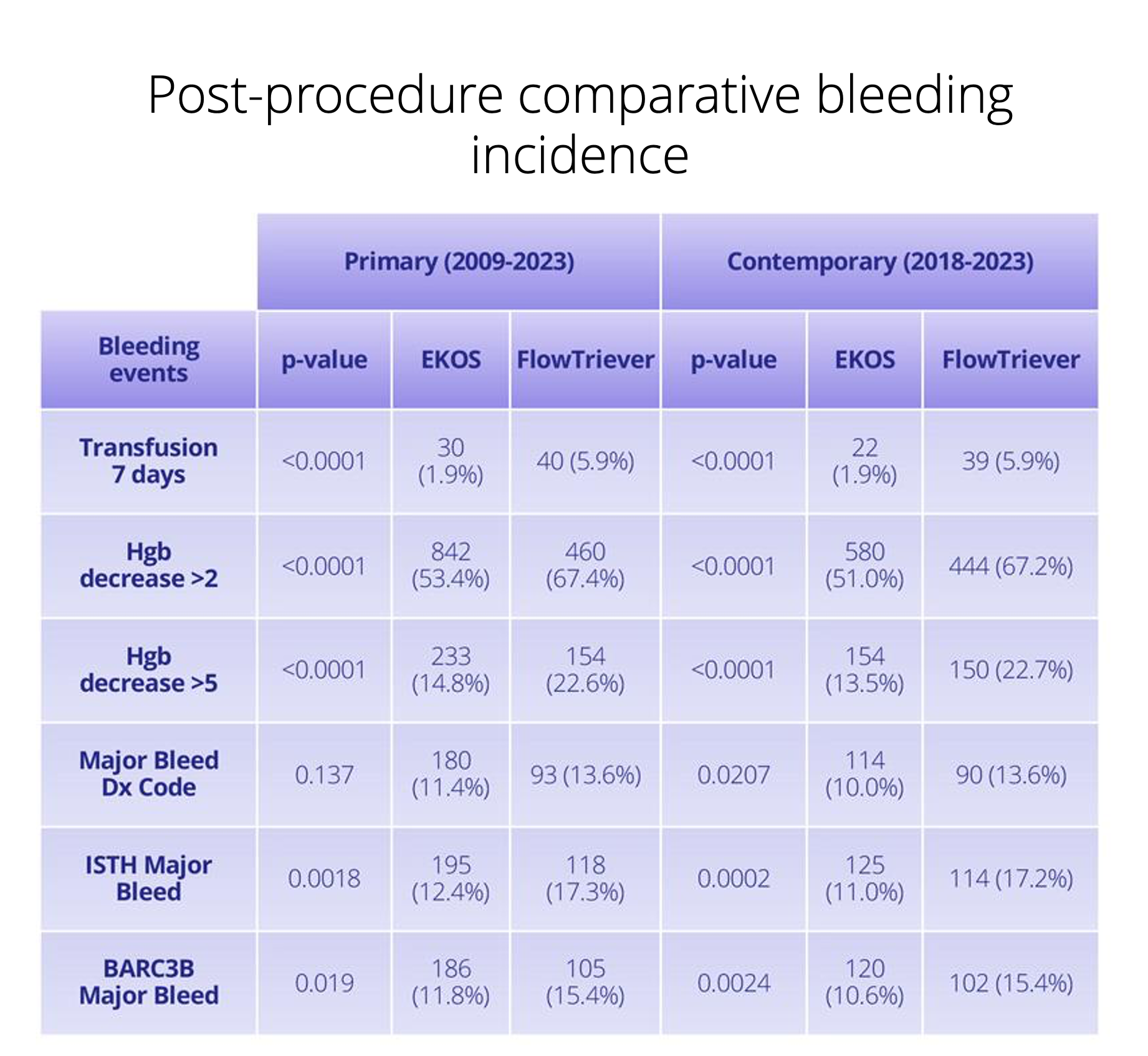
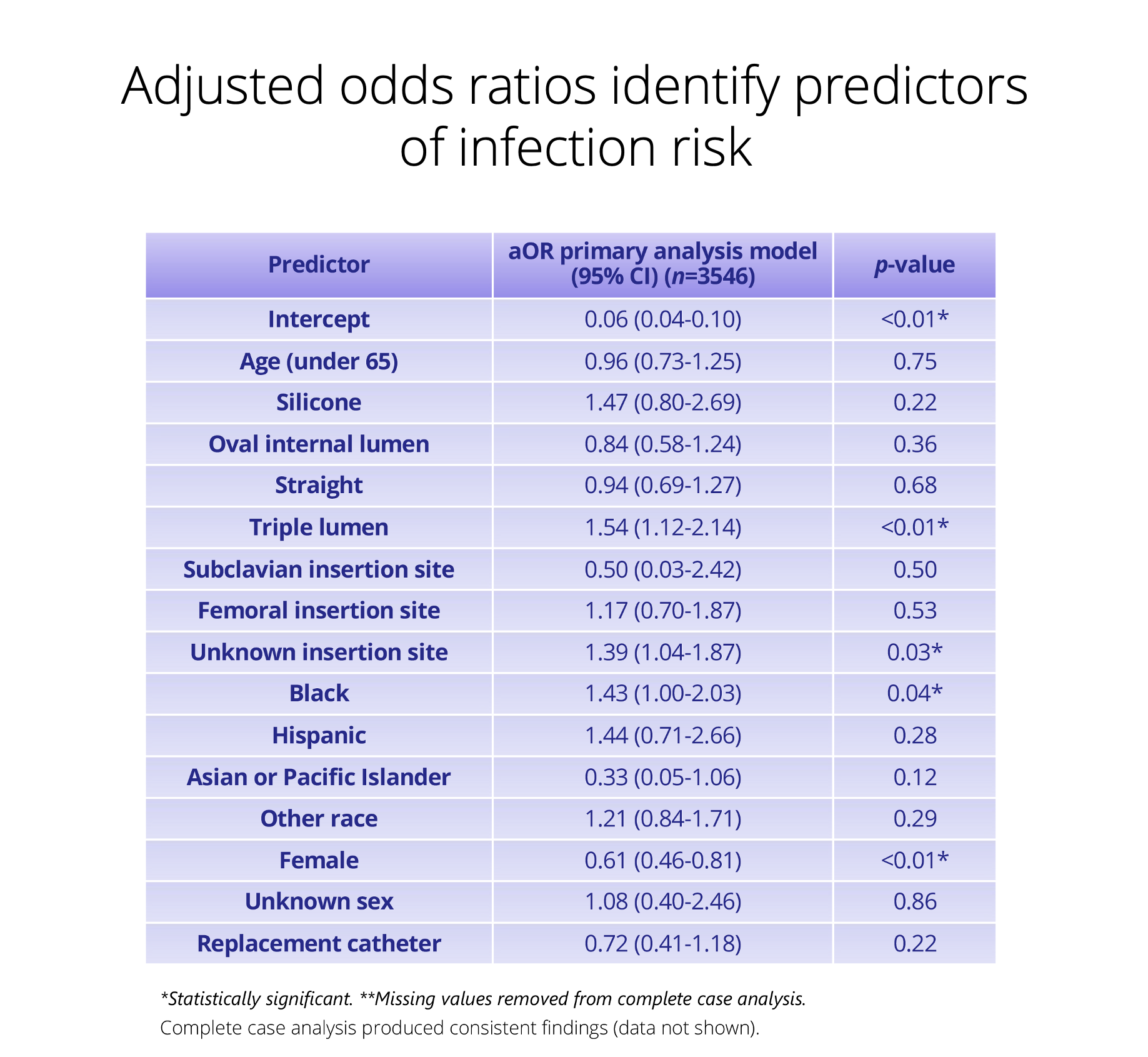
Support regulatory submissions and post-approval commitments with fully traceable, auditable real-world data.

Monitor safety signals during and after studies with daily refreshed data spanning care settings.