
by Truveta staff | Apr 25, 2024 | Data, News
Today, we are excited to announce the largest and most complete mother-child electronic health record (EHR) dataset for scientifically rigorous research on mothers and their children. Truveta empowers researchers with unparalleled insights into the continuum of care...
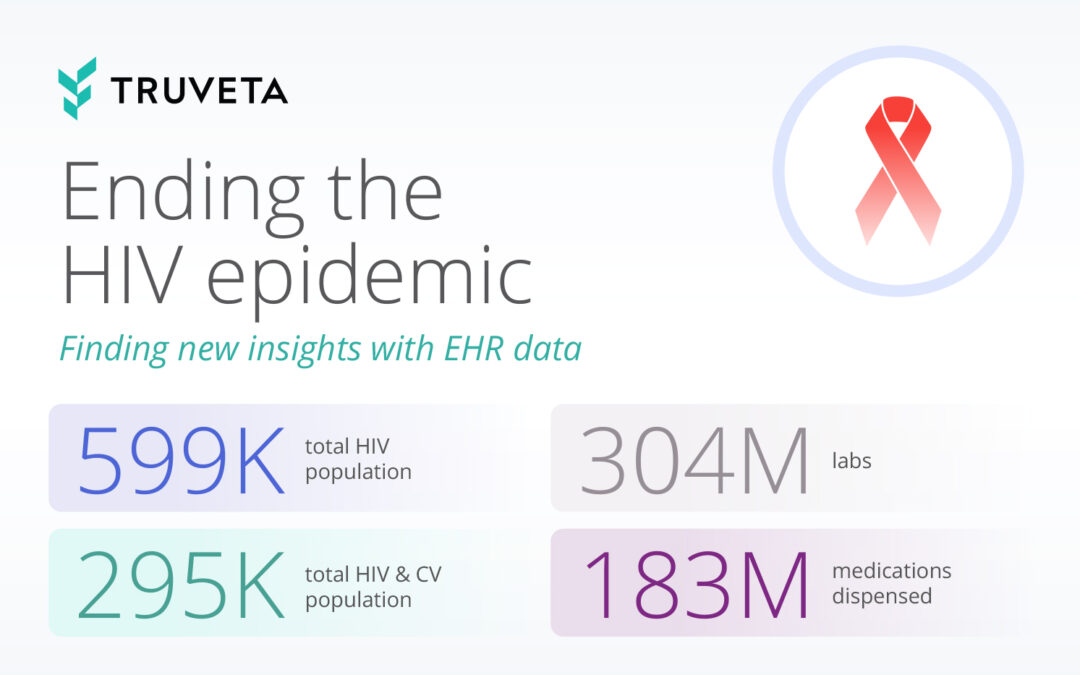
by Truveta staff | Apr 18, 2024 | Data
In 2019, the US government unveiled the ambitious Ending the HIV Epidemic (EHE) plan, aiming to slash new HIV infections by 90% by 2030. Thanks to advances in antiretroviral therapy (ART), proven models of prevention, and data access, new HIV infections declined 12%...

by Truveta staff | Apr 4, 2024 | Data
US spending on medical devices and in-vitro diagnostics totals more than $199 billion a year, with most of the costs associated with clinical development. Label expansion provides a pathway for recouping costs associated with the device development process by...

by Truveta staff | Apr 2, 2024 | Data
A common misconception is that drugs and devices must gain explicit approval from the FDA for each specific use before healthcare providers can employ them. However, a practice known as off-label use challenges this notion, revealing a broader landscape of treatment...
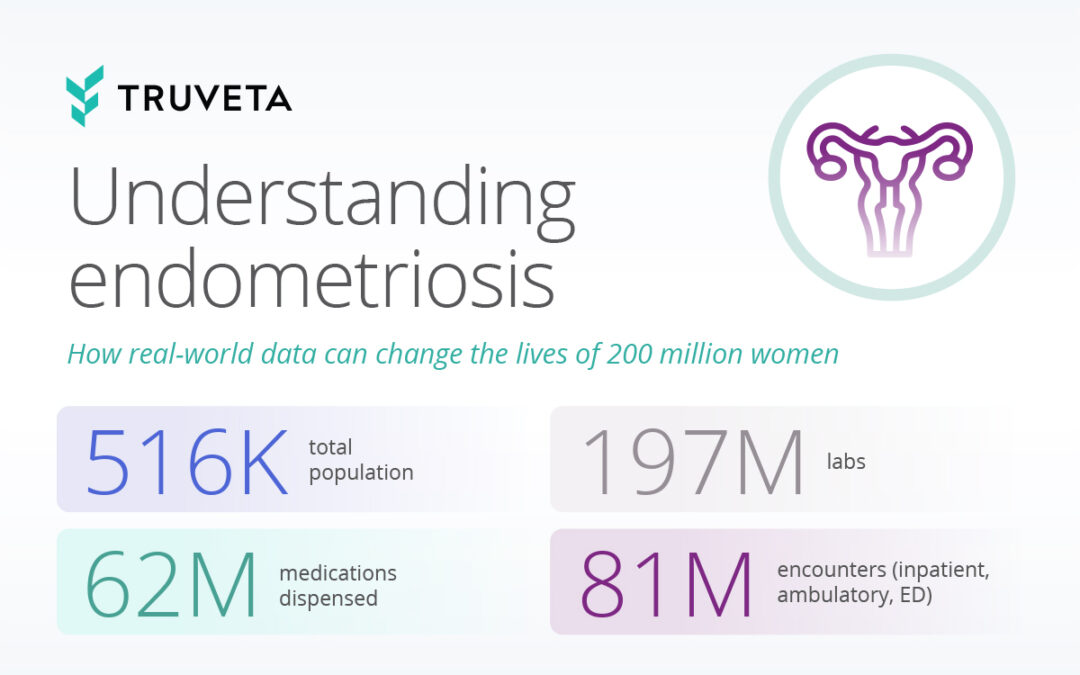
by Truveta staff | Mar 12, 2024 | Data
Stories from women whose endometriosis pain has been dismissed are devastatingly common. A typical woman in the United States will wait 10 years after her symptoms first appear to receive an endometriosis diagnosis. This delay is rooted in a pervasive lack of...
by Truveta staff | Feb 29, 2024 | Data, News, Technology
Healthcare data traditionally has been too siloed, inaccessible, and messy to be useful for research. And one of the biggest challenges has been that critical information about a patient’s health is locked away in the unstructured text in clinician notes. Thanks to...







