Infections remain one of the most concerning complications following ureteroscopy (URS), a common procedure to examine or treat kidney stones. Even though URS is considered a safe and routine procedure, postoperative infections can lead to serious outcomes, including sepsis. Increased infection risk has been associated with intrarenal pressure elevation that leads to intrarenal backflow. To evaluate whether new pressure monitoring device features could improve patient safety, Boston Scientific collaborated on a study using Truveta Data to compare infection outcomes across single-use ureteroscopes. With unique device identifier-level detail, researchers were able to precisely link patient outcomes to the device used, enabling a direct comparison.
Study snapshot
Boston Scientific researchers in collaboration with Université de Montréal and University of California San Diego School of Medicine conducted a study using Truveta’s EHR data comparing the LithoVue™ Elite Single-Use Digital Flexible Ureteroscope (LVE) with intrarenal pressure monitoring against other single-use ureteroscopes. The study evaluated:
- 208 patients treated with LVE
- 416 patients treated with other single-use ureteroscopes
Key findings at 30 days post-procedure included:
LVE use was associated with significantly lower rates of post-operative infections (8.2% vs. 15.4%).
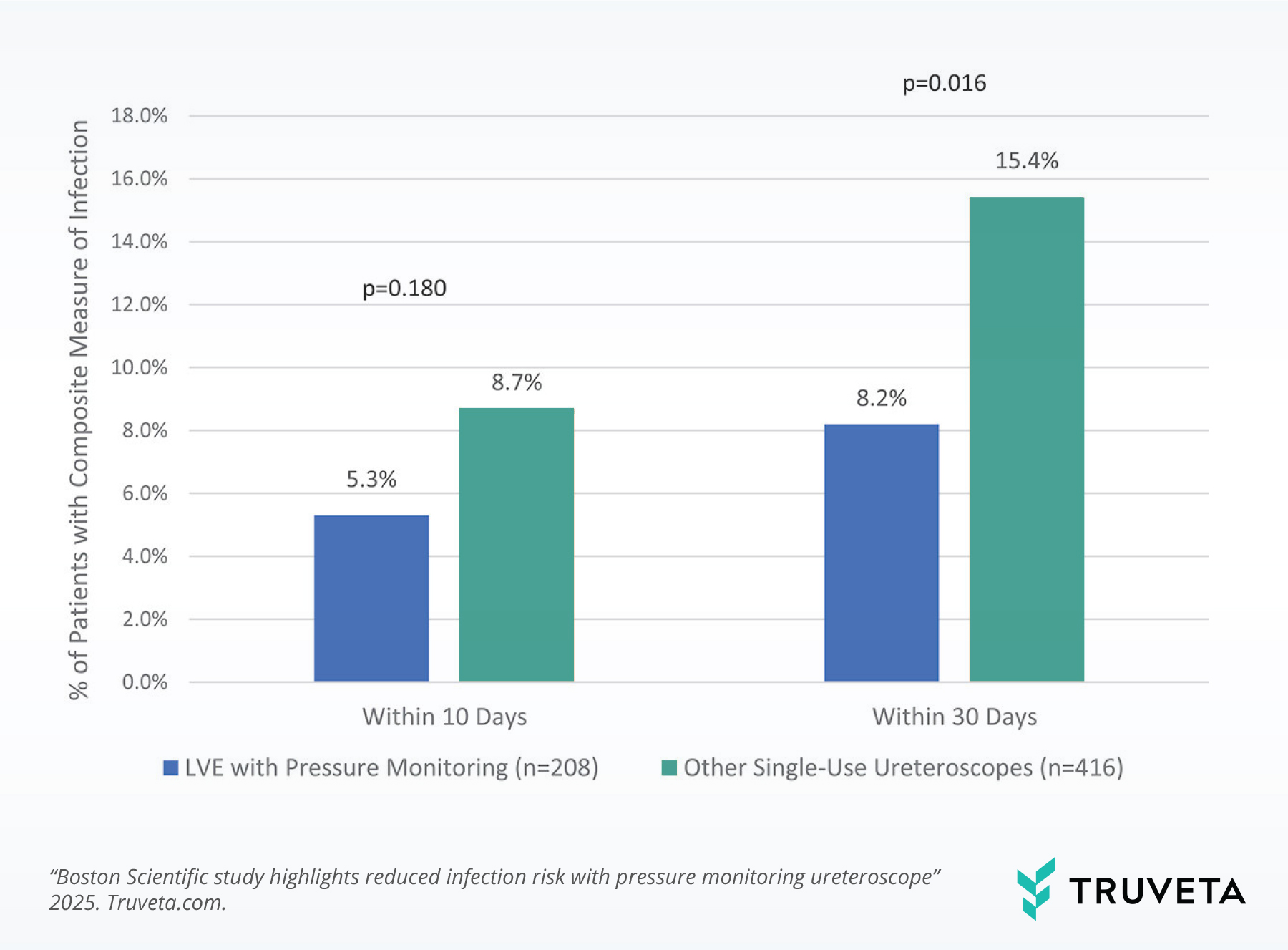
LVE use was associated with half the rate of post-operative Urinary tract infections (UTIs) (5.2% vs. 10.8%).
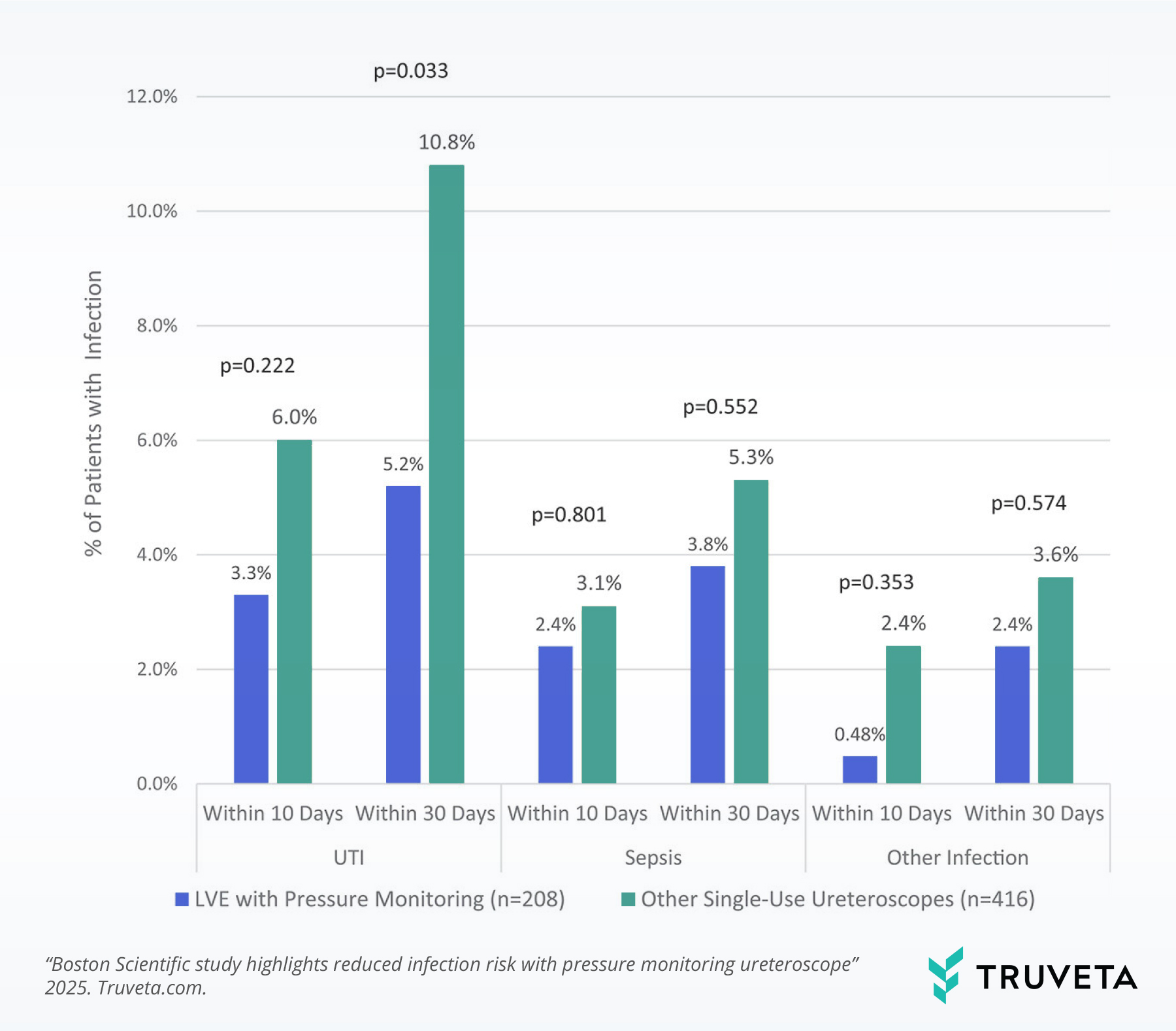
Patients treated with other single-use ureteroscopes had more than double the odds of infection.
Why it matters
These results highlight the potential of intrarenal pressure monitoring use to improve safety in URS procedures. For clinicians, fewer infections mean better patient outcomes and lower complication rates. For health systems, reductions in infection translate into fewer readmissions and lower costs. For device manufacturers, being able to show reduced infection risk at scale can help accelerate adoption.
As medical devices continue to evolve, generating high-quality, real-world evidence at the unique device identifier level is essential.
Answers can’t wait. Let’s talk about how Truveta can accelerate device innovation.

