In this Truveta experts spotlight, Jared Kern and Vidya Venkataraman, PhD explored trends in the use of Rituxan biosimilars between 2018 and 2024. Their analysis of Truveta Data reveals how patient access and switching behavior has evolved over time.
Jared Kern is a Research Analyst on Truveta’s Partner Research and Success (PRS) team. Jared is a data scientist & engineer and has a background in providing advanced analytics across clinical R&D teams at several federal health agencies.
Vidya Venkataraman, PhD is a Partner Success Manager on Truveta’s PRS Team. Vidya is a health economist and has a background in HEOR, cost and quality measurement, and value-based care.
To explore how biosimilars are being used in clinical practice and how quickly they may be replacing biologic predecessors, Jared and Vidya analyzed real-world adoption of Rituxan biosimilars across more than 20,000 patients from 2018 to 2024, spotlighting key trends in switching, access, and uptake across patient populations.
What are biosimilars, and why do they matter?
The U.S. biosimilar market continues to evolve—driven by expanded regulatory approvals, payer incentives, and efforts to improve affordability. Biosimilars are highly similar, but not identical, versions of the original biologic / bio-originator drug. Biosimilars can often offer a lower-cost alternative, aiming to improve access without compromising care, efficacy, or safety.
One of the most widely used biologics is Rituxan (rituximab)—a monoclonal antibody that targets CD20 on B cells to treat a range of hematologic cancers and autoimmune conditions. Rituxan (rituximab) is approved for:
- Non-Hodgkin lymphoma (NHL)
- Chronic lymphocytic leukemia (CLL)
- Rheumatoid arthritis
- Granulomatosis with polyangiitis (GPA)
- Microscopic polyangiitis (MPA).
Rituxan biosimilars—Truxima, Ruxience, and Riabni—were FDA-approved between 2018 and 2020 and are now in wide use.
Still, real-world adoption has varied across drugs and providers and patterns of switching from originator drugs to biosimilars is an area ripe for further investigation using real-world data. A recently conducted study on Humira (adalimumab) and biosimilars by Truveta Research showed that while the use of biosimilars was on the rise, 1 out of 8 patients switched back to the original bio-originator drug.
We wanted to understand how Rituxan’s biosimilars were adopted in the real-world: Was the adoption of biosimilars following their approval by the FDA immediate? Were certain demographic groups—based on payer type — more likely to switch? And what might these patterns reveal about real-world access and decision-making in cancer and autoimmune care?
Methods
We analyzed the real-world use of Rituxan and its biosimilars across more than 20,000 patients between 2018 and 2024 using a subset of Truveta Data. All patients in our study had received at least one administration of Rituxan or one of its biosimilars (Truxima, Ruxience, Riabni), we refer to this population as treated patients.
Patient treatment exposure
We describe the yearly proportion of patients who received Rituxan or one of the biosimilars. Because patients could receive both Rituxan and a biosimilar in the same year, these treatment groups are not mutually exclusive—reflecting overall exposure rather than exclusive use. Separately, we describe the proportion of patients who exclusively received Rituxan or biosimilars, and those who received both within one calendar year.
Trends in proportion of treated patients over time
We calculated the monthly proportion of treated patients by drug and applied a 3-month moving average.
Biosimilar adoption by coverage type
We were particularly interested in understanding how patients transitioned between these therapies over time—and whether this varied by insurance type.
Results: What Truveta Data show
Patient treatment exposure
In 2018, over 90% of treated patients received Rituxan. However, that dominance declined quickly following the FDA approval of biosimilars. By 2020, biosimilars accounted for treatment in nearly half of all patients and the proportion continued to increase, reaching nearly 70% in 2024.
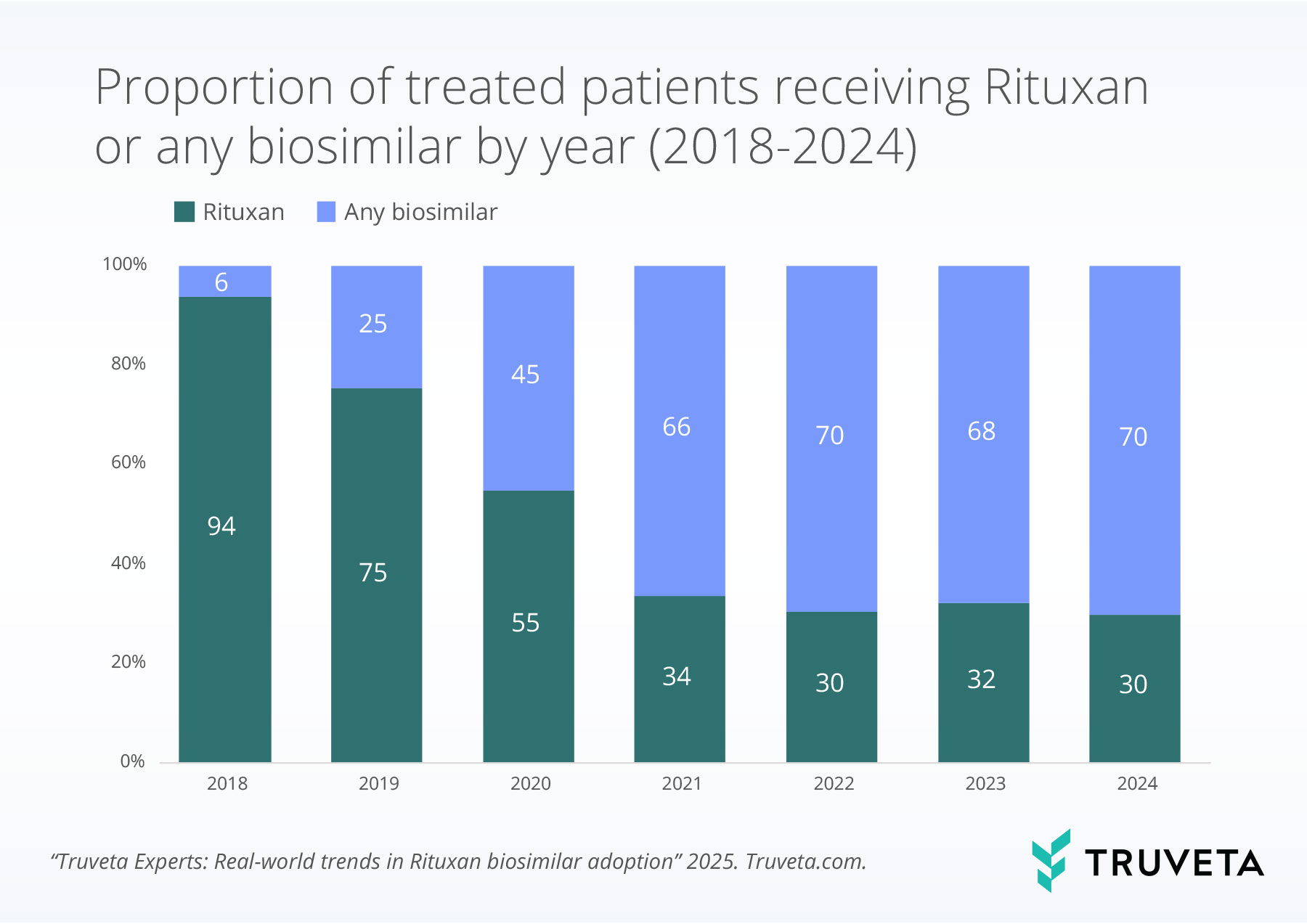
Trends by treatment category
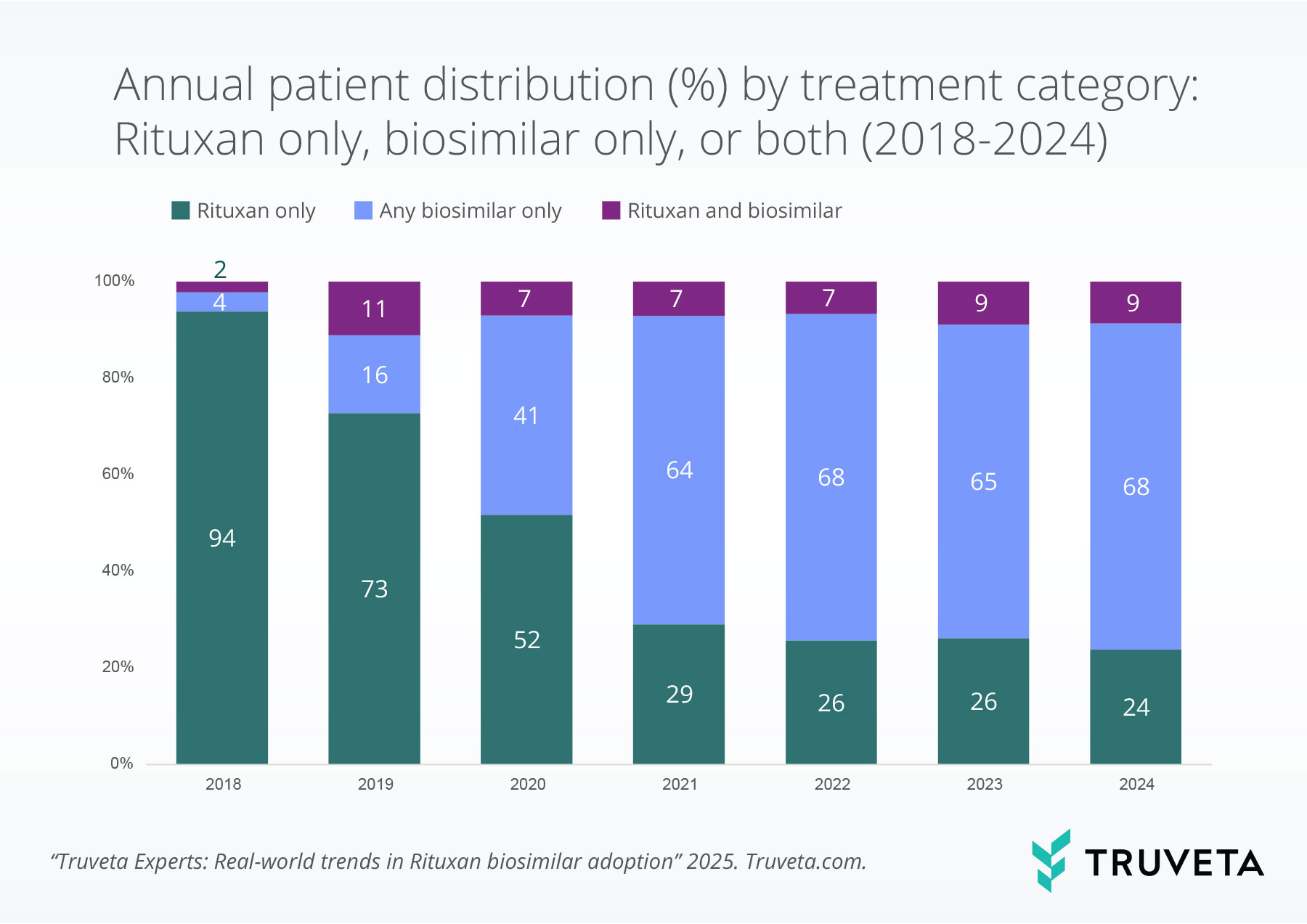
Most patients were administered only Rituxan or a biosimilar within each year. In 2018, 94% of patients received Rituxan only, but by 2024—after all three biosimilars had entered the market—that proportion dropped to 24%. Meanwhile, biosimilar-only use grew to 68%, signaling a clear shift toward biosimilar adoption. and biosimilar use was highest in 2019, presumably when patients may have originally switched from Rituxan to a biosimilar.
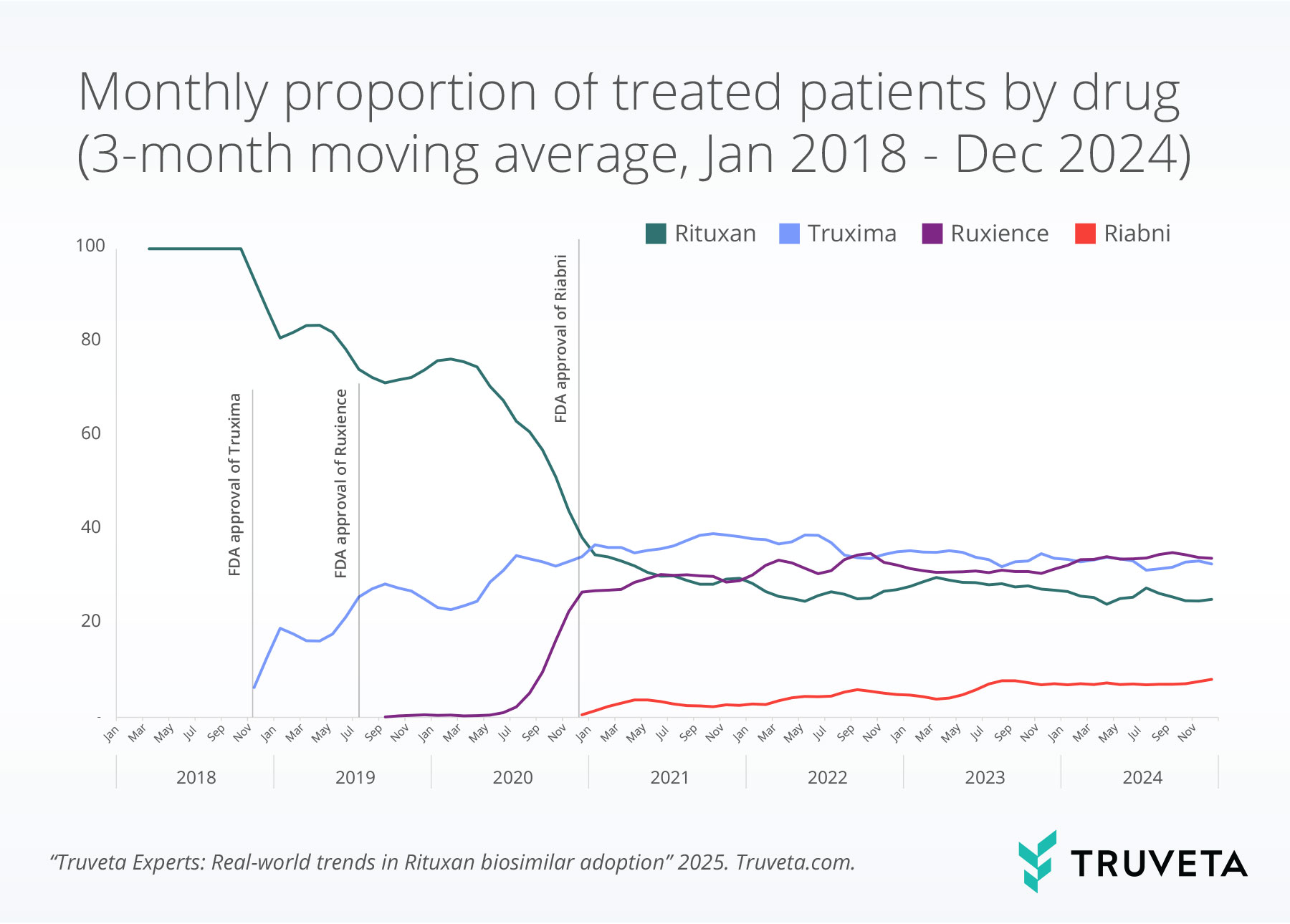
Trends in proportion of treated patients over time
By early 2021, biosimilars collectively surpassed Rituxan in proportion of use and remained the predominant treatment category throughout the rest of the observation period.
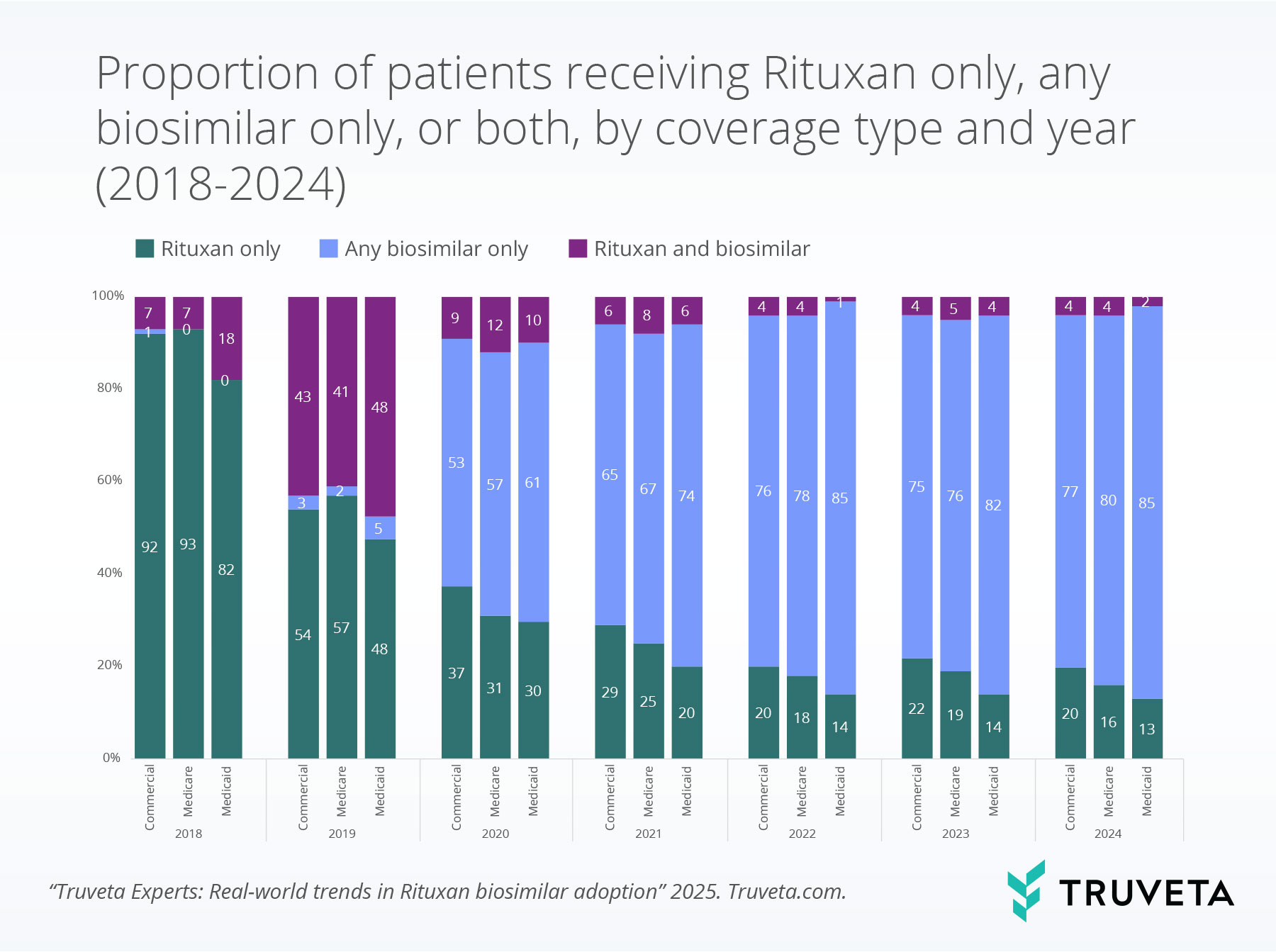
Biosimilar adoption by coverage type
By 2024, patients with Medicaid coverage had the highest proportion exclusively receiving biosimilars, suggesting earlier or more complete transitions in this group compared to those with commercial or Medicare coverage.
Discussion
Biosimilars are increasingly used in real-world practice—but switching patterns may remain nuanced. Our findings show a steady transition away from Rituxan toward biosimilars, yet this adoption varies slightly by coverage type—signaling a potential interplay between access, cost, and care delivery that can be further explored.
“Over the next decade, multiple biologic therapies are expected to have their patent protections expire, paving the way for new biosimilar development, adoption, and competition. This shift will not only pave the way for further studies on the safety, efficacy, and effectiveness of biosimilars, but also raise important questions about their impact on healthcare costs and quality of care. As biosimilars enter broader clinical use, real-world data will play a critical role in monitoring their uptake, understanding switching patterns, and evaluating their performance across diverse patient populations and healthcare settings.” Vidya Venkataraman, PhD
Limitations
Understanding biosimilars adoption by payer type is important to address potential equity and access challenges. However, there are certain limitations associated with this analysis. Some patients may have had multiple coverage types within a calendar year and thus in this analysis would be counted in each of the payer categories. Further, the payer types included in this analysis are not an exhaustive list but represent the most common or highest distribution of patients.
Future Research
As more biologics lose exclusivity, biosimilars will continue to expand across therapeutic areas. This raises several questions for future exploration using real-world data:
- How do formulary mandates and step therapy shape biosimilar use?
- What role do social drivers of health (SDOH) play in adoption?
- Are there differences in outcomes between those who switch vs. those who don’t?
- What explains switching back to the originator?
- Are there cost savings associated with switching?
About Truveta experts
The Truveta experts series highlights insights from researchers, clinicians, and data scientists working across Truveta. Each post highlights how real-world data can unlock new understanding—from disease patterns to treatment complexity to population health trends.
Explore more in this series:
Treatment trends for trigeminal neuralgia
Fireworks during the fourth of July
Real-world trends in Rituxan biosimilar adoption
Sudden cardiac arrest and arrhythmias among patients with chronic kidney disease
Biomarker testing trends in metastatic non-small cell lung cancer
See how Truveta Data can accelerate your next study—Request a customized demo today
These are preliminary research findings and not peer reviewed. Data are constantly changing and updating. These findings are consistent with data accessed July 2025.
References
- Mamori T, Tanioka M, Takada K, et al. Real-world comparative analysis of trastuzumab originator and biosimilars: Safety, efficacy, and cost effectiveness. BioDrugs. 2024;39(1):131–142. doi:10.1007/s40259-024-00686-x
https://link.springer.com/article/10.1007/s40259-024-00686-x - Mulcahy AW, Hlavka JP, Case SR. Biosimilar cost savings in the United States: Initial experience and future potential. Rand Health Q. 2018;7(4):3. PMID: 30083415; PMCID: PMC6075809.
https://pmc.ncbi.nlm.nih.gov/articles/PMC6075809/ - Herndon TM, Ausin C, Brahme NN, et al. Safety outcomes when switching between biosimilars and reference biologics: A systematic review and meta-analysis. PLoS One. 2023;18(10):e0292231. doi:10.1371/journal.pone.0292231
https://journals.plos.org/plosone/article?id=10.1371/journal.pone.0292231
