See real-world impact sooner with detailed, real-time data
Support R&D, safety surveillance, and label expansion with representative, complete and timely device data that exceeds FDA standards for data quality, data provenance, and audit readiness.
Reach out and we’ll schedule a meeting
Access complete, research-ready data for every step of the device lifecycle
From pre-market development through post-market surveillance, Truveta provides the most complete real-world data available — enabling your teams to evaluate device performance, improve outcomes, and accelerate innovation.
EHR data from more than 120 million patients, including clinical notes and images, linked with closed claims from more than 200 million patients.
Read how real-world data is replacing trials and registries
patients
patients linked with closed claims
unique devices
years longitudinal patient data
clinical notes
imaging studies
Data are de-identified, updated daily, and audit-ready
Read how real-world data is replacing trials and registries
Regulatory-grade safety and effectiveness data
What you can do with Truveta

Confidently compare devices across diverse, representative populations in weeks, not years


Monitor safety signals in real time, across care settings

Generate evidence to support label expansion and payer reimbursement

Analyze UDI-level data to study outcomes such as:
- In-hospital mortality
- 30-day readmissions
- Major adverse events
- Length of stay
Case study

Boston Scientific used Truveta to compare the EKOS™ Endovascular System to Inari’s FlowTriever, revealing a higher bleeding risk with the competitor. The study was published and presented within 5 months, saved years and millions that it would have taken to complete a RCT, and improved adoption of the EKOS(TM) Endovascular System.

Real-world data to support your next indication - faster than you thought possible
Expand access to your therapy by showing value in the real world. With Truveta, you can analyze diverse patient populations and care settings to demonstrate broader effectiveness and cost savings.
Build your case for:
- Payer reimbursement
- Indication expansion
- Off-label usage validation
Truveta Data in action
A cardiovascular device manufacturer used Truveta Data to demonstrate improved long-term outcomes in high-risk patients – accelerating payer engagement and supporting label expansion efforts. With commercial, Medicare, and Medicaid claims linked to EHR data, real-time visibility into off-label use, and comprehensive longitudinal records, Truveta offers the real-world evidence needed to expand access and validate broader indications.
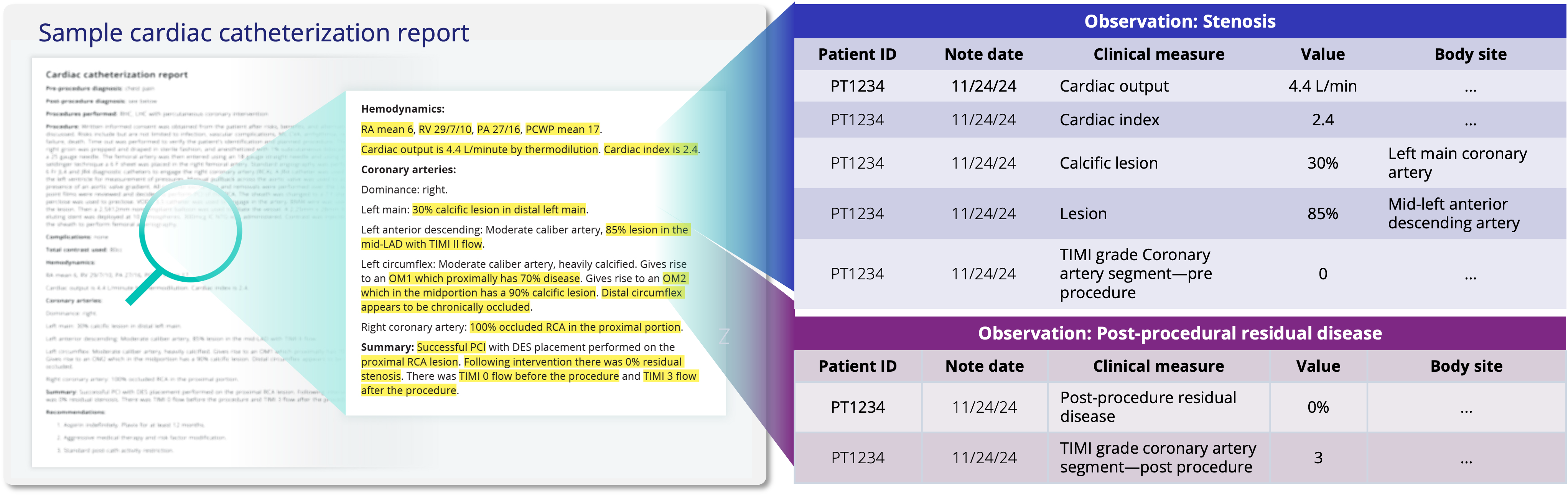
Clinical notes reveal what structured data misses
Truveta captures 7B+ clinical notes – including progress notes, operative reports, discharge summaries, and more – offering insight into treatment rationale, complications, and adverse events.
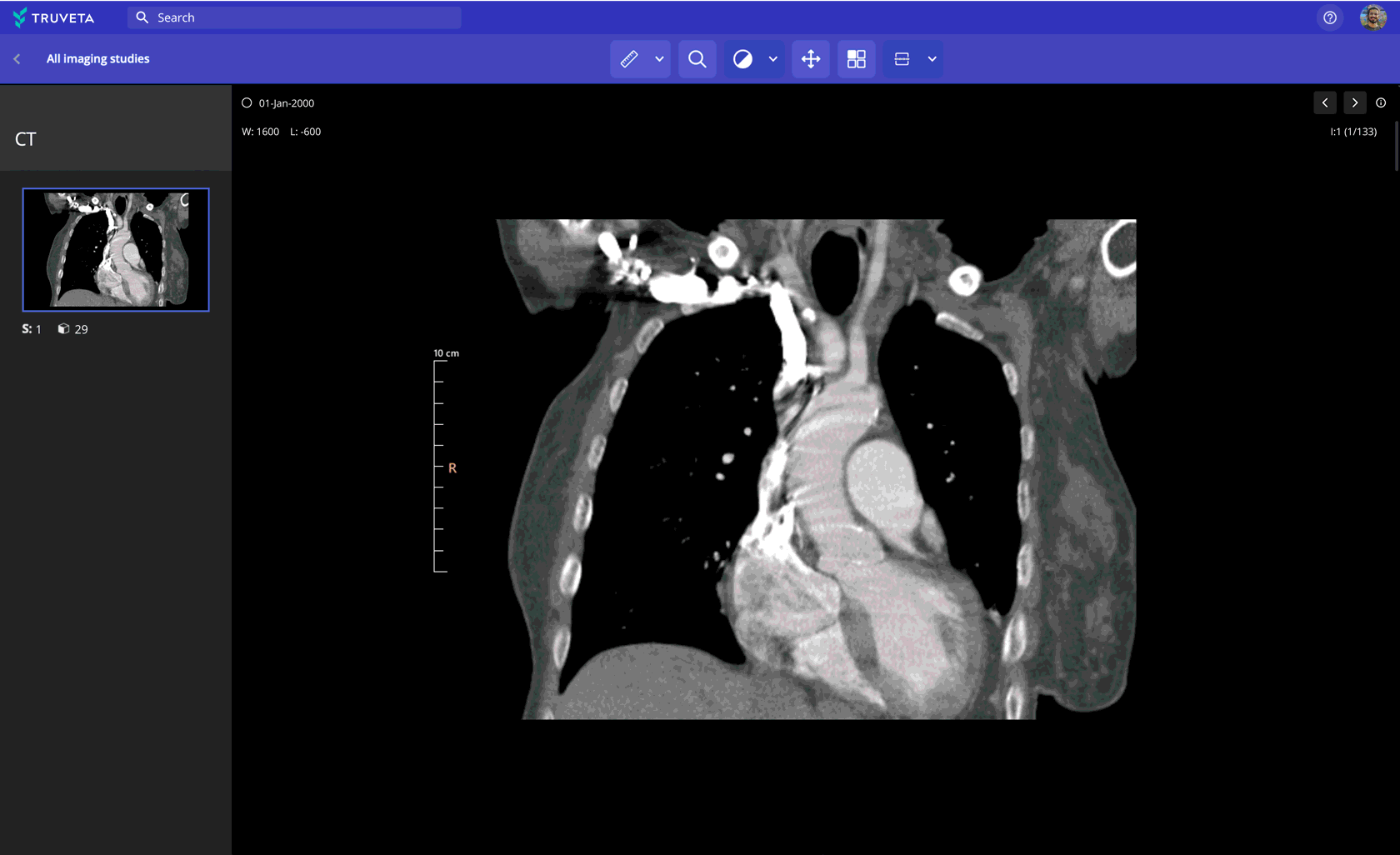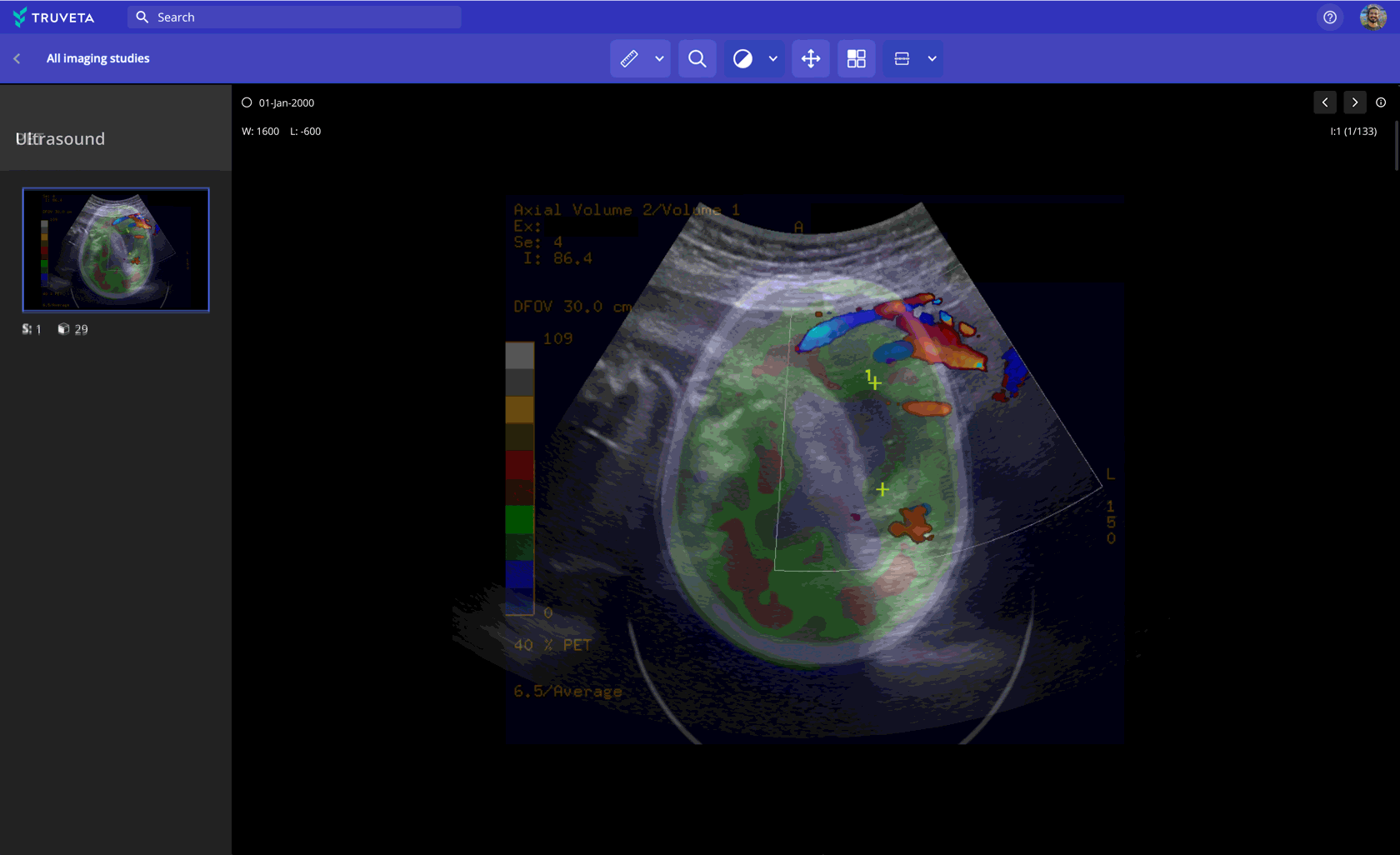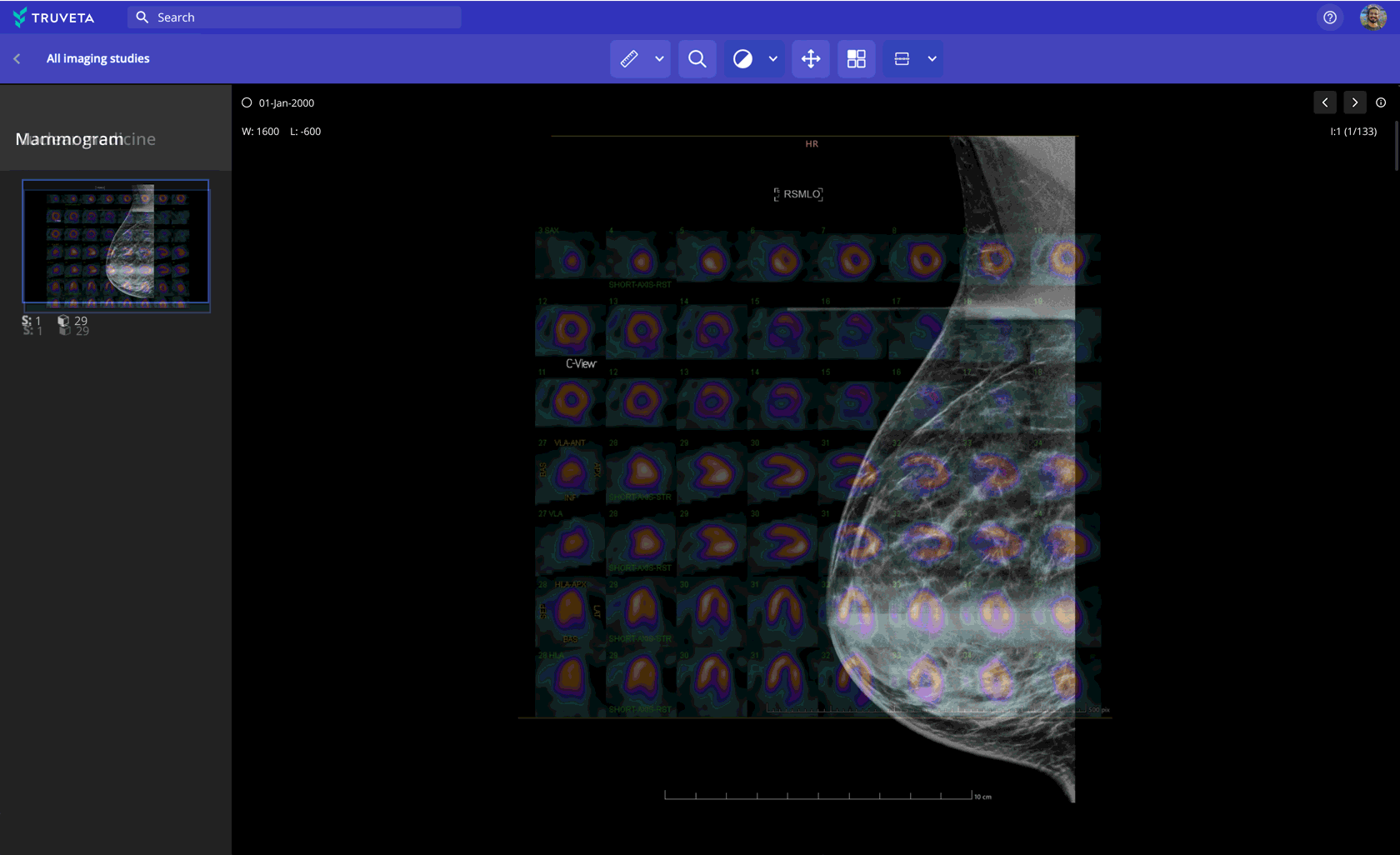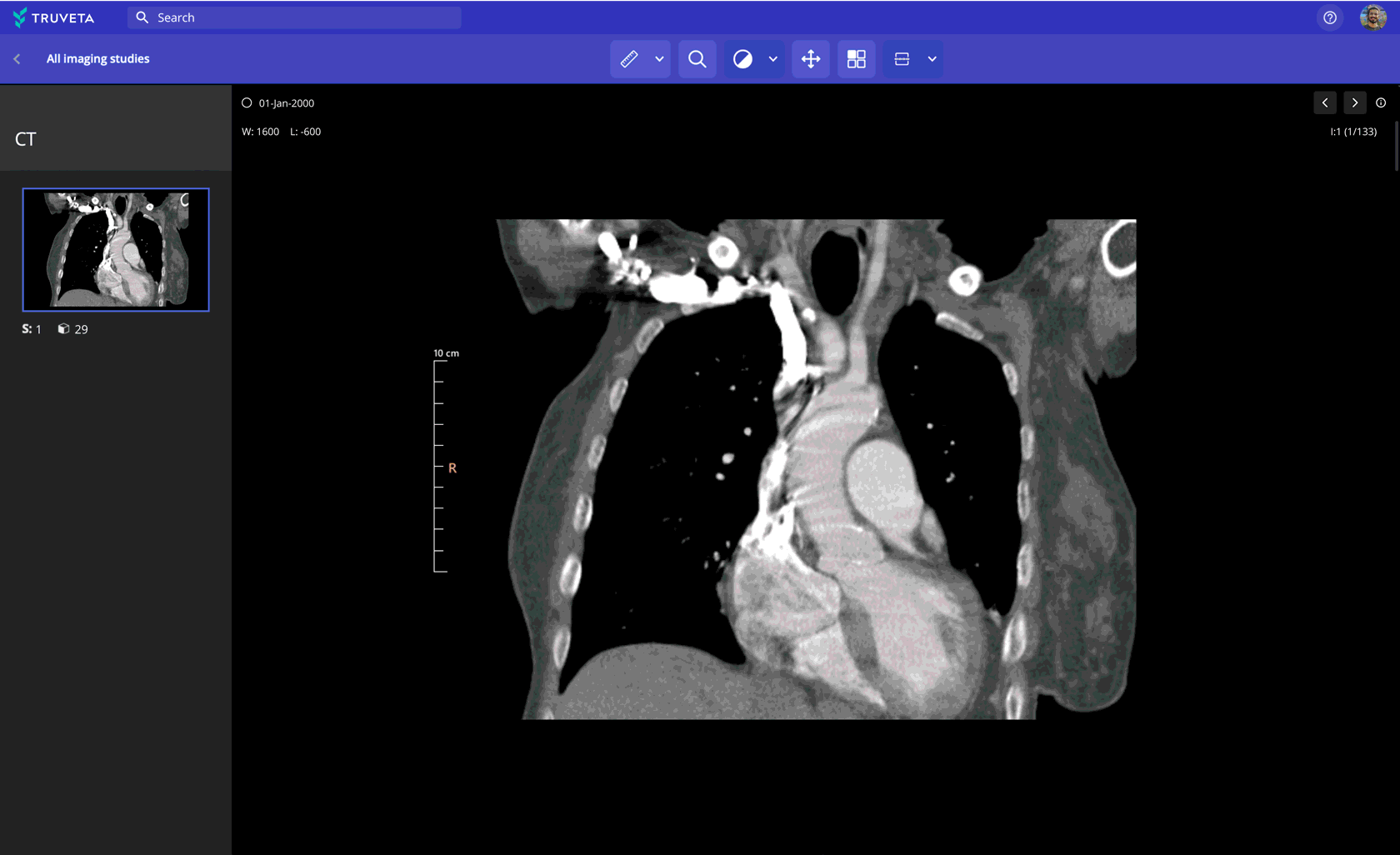
Imaging data paired with EHR and claims
Leverage 100M+ medical images (CT, MRI, X-ray, and more) to:
- Assess device performance and anatomical change over time
- Support multimodal AI model development
- Analyze recovery trajectories and safety indicators
Device innovation across specialties
Use Truveta Data to:
Compare bleeding risks and long-term outcomes in embolism treatments
Analyze treatment patterns and safety signals for neurostimulators
Evaluate implant performance and surgical outcomes with integrated imaging and clinical notes
Connect remote monitoring device data with EHR outcomes for predictive modeling
No matter your device category, Truveta’s real-world evidence helps you move faster, prove value, and improve care.
Advance your research with Truveta
Contact us today for a demo and see how Truveta’s data can power your medical device research.