Real-world evidence is critical for understanding how therapies perform beyond controlled clinical trials. Yet many life sciences organizations rely on fragmented proprietary data that lack the longitudinal clinical context needed to support regulatory, payer, and product decisions—limiting visibility into outcomes, utilization, and safety over time.
Unifying fragmented data with Truveta Live Link
Live Link facilitates secure and privacy-preserving linkage of proprietary first-party datasets into longitudinal clinical and claims data within a single, self-governed environment. Rather than relying on one-time or episodic integrations, Live Link establishes a sustained, continuously updating connection. As new data accrue, linked cohorts remain intact as patients transition from formal study participation into routine care—preserving continuity without repeated re-linking or siloed data workflows.
The value of this live linkage comes from its connection to Truveta Data—daily refreshed, regulatory-grade EHR data spanning more than 130 million patients, linked to claims, mortality, and social drivers of health. Because clinical data update continuously as care unfolds, researchers can define cohorts and endpoints in advance and follow patients forward over time.
Together, persistent linkage and daily refreshed longitudinal data enable a new class of prospectively designed real-world research. Organizations can support sustained longitudinal follow-up beyond individual studies, optimize completeness for regulatory- and payer-grade evidence, and observe outcomes, utilization, and safety signals under contemporary standards of care—without rebuilding data pipelines for each analysis.
This continuously updating evidence foundation underpins prospective evidence generation across the product lifecycle, from clinical development and HEOR to safety monitoring, unmet need assessment, and ongoing R&D.
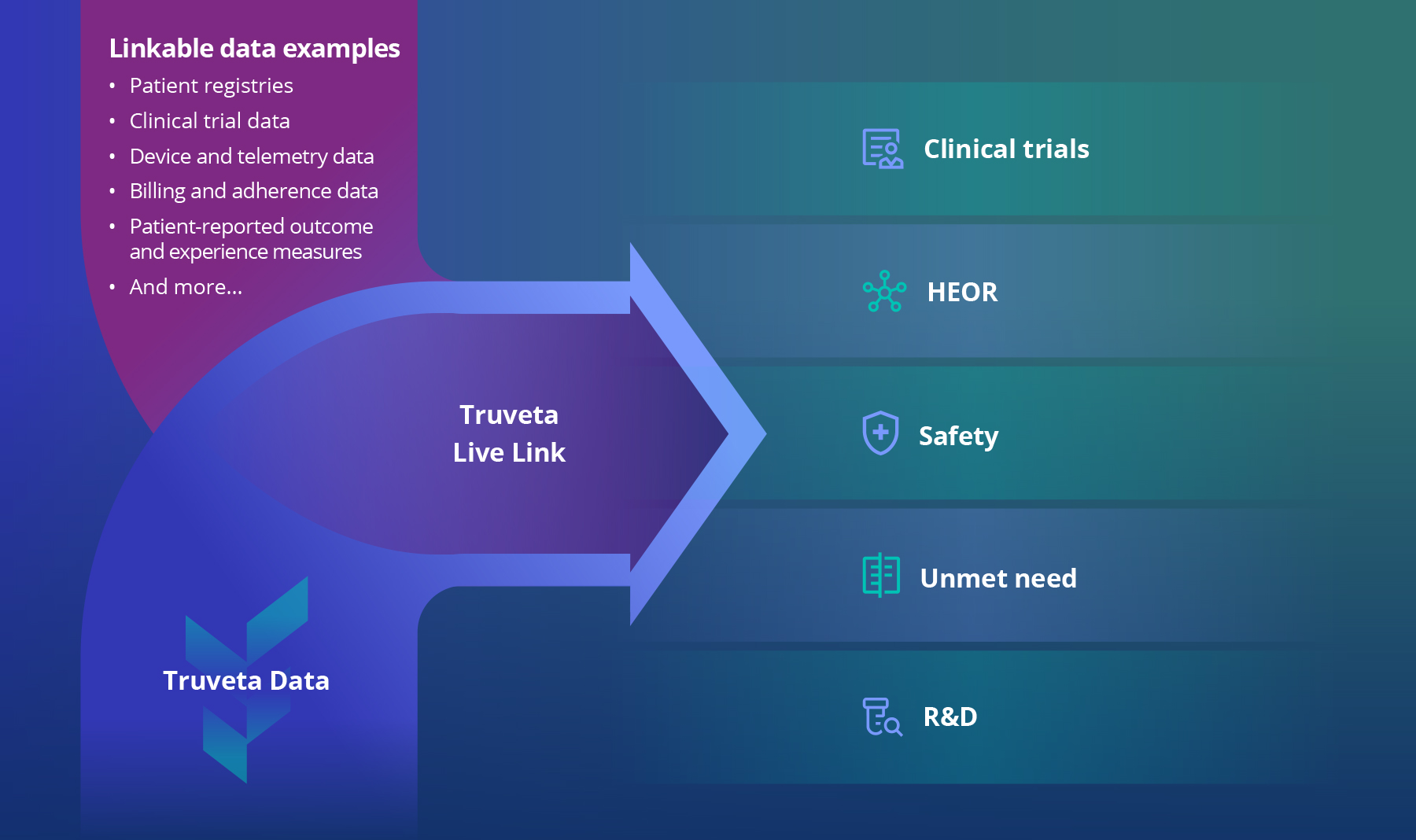
Truveta Live Link creates a continuously updating, longitudinal evidence foundation by linking proprietary data with real-world clinical context.
By unifying fragmented proprietary data within a continuously updating longitudinal framework, Truveta Live Link converts real-world data into a strategic evidence asset—enabling prospective understanding of real-world use, performance, and outcomes at population scale.
To learn more about how organizations use Live Link across the product lifecycle, download the white paper, Truveta Live Link: Enabling complete, prospective real-world evidence at scale.

