Today in a keynote at ViVE, I unveiled the Truveta platform for the first time with its first public demonstration. While preparing for this keynote, I reflected on how far our team, and our members, have come in the last two years, amidst the backdrop of the COVID-19 pandemic.
In early 2020, Providence discovered the first U.S. case of COVID-19, and then the World Health Organization (W.H.O.) declared COVID-19 a global pandemic. Thousands of people were dying. Schools and workplaces transitioned abruptly to fully remote. Our expectations were set that vaccines would take many years to develop. Many of us feared for ourselves and our loved ones. President Trump and the W.H.O were debating medical facts on Twitter, without any underlying data being available to support either position. It was a tense time full of uncertainty but led to the creation of Truveta. Innovative health system leaders came together to share their data and build an unprecedented data platform that would make it easier to ask and answer questions about American health.
As part of our Truveta demo, we showed how any researcher in the Truveta community can easily reproduce our November clinical study on COVID-19 breakthrough infections and co-morbidities. Our research team reproduced the study themselves with updated data, and just this past week, found new insights with regards to the Omicron wave.
Offering unprecedented data
Healthcare data is fragmented, unstructured and fraught with privacy concerns. Researchers struggle to access data that is current and representative of the populations they serve. Truveta’s platform empowers researchers with an unprecedented data platform – including 16% of clinical care in the US from its 20 health system members, all carefully de-identified, representing the full diversity of our country across age, geography, race, ethnicity, and sex. This clinical data provides the scale to be representative of our country and to find the needles of insights in the haystacks of data to help improve patient care. It is updated daily and continuously flowing to be responsive in pandemics, quickly staff clinical trials, and to provide the most up-to-date picture of US health. Data includes the complete medical records captured during care (including clinical notes, images, and genomics), not just the medical bill from claims data.

To complete data on the patient journey, Truveta partnered with LexisNexis Risk Solutions. This partnership enables Truveta to link medical records across providers (reliably identifying individuals across name changes and name misspellings), provides mortality data on the 2/3 of people who die outside of the hospital, and provides claims data on when care is received outside of Truveta’s members (70% of all commercial medical insurance claims, claims for 40% of Medicare and Medicaid patients). LexisNexis Risk Solutions also provides Truveta socioeconomic on every patient, a key driver of health outcomes and enabling the study of health inequities. Together, we’re building the most complete, highest quality U.S. public health data platform.
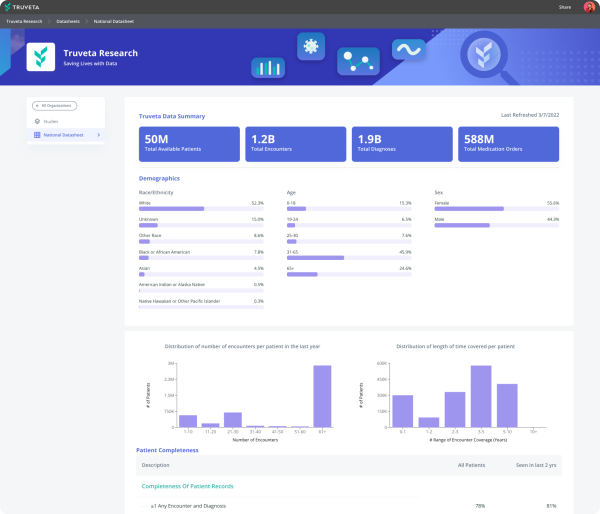
We normalize data for faster insights using AI and carefully de-identify data compliant with HIPAA, while retaining patient journey timelines. Once de-identified, aggregate data flows into the Truveta platform where researchers have many options to analyze the data, including viewing in customizable daily dashboards, analyzing within Jupyter notebooks, or exporting into their preferred analysis tools.
It was my honor to represent our 20 health system members and show the Truveta platform for the first time today.
New real-time insights on COVID-19
As we build Truveta for all research into all diseases, our Truveta Research team is focused on COVID-19. The team used the Truveta platform to build COVID-19 dashboards tracking breakthrough cases, vaccine effectiveness, and outcomes like hospitalization every day across the US. The dashboard is interactive, allowing researchers to select populations to study by age, sex, race, comorbidity and vaccine dose and manufacturer. It was exciting to share this work from our research team and the potential of the underlying platform at ViVE.
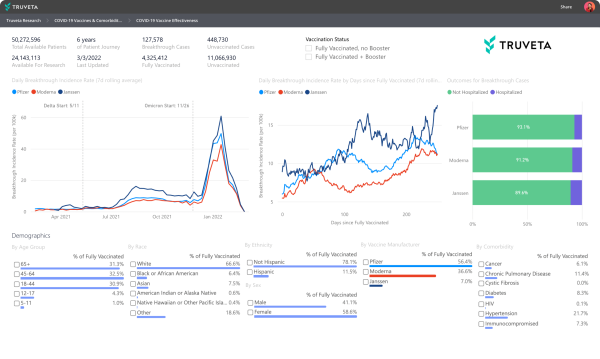
Within the COVID-19 dashboard, we include data to represent COVID-19 infections and hospitalizations for those patients with co-morbidities like diabetes, cancer, and hypertension, just to name a few.
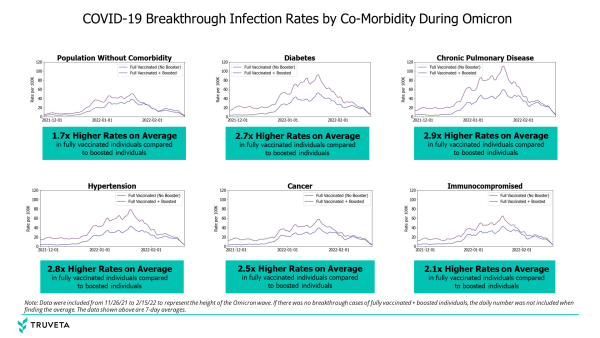
Today, I’m excited to share new insights the Truveta Research team discovered specific to COVID-19 breakthrough rates among fully vaccinated and boosted patients with co-morbidities during the Omicron surge. The Truveta researchers found that independent of disease status, patients who were boosted had lower rates of breakthrough infections than patients who were fully vaccinated.
- People without comorbidities who were vaccinated and not boosted were 1.7 times more likely on average to have a breakthrough infection than those who were boosted
- People with a range of co-morbidities were more likely to have breakthrough infections if not boosted, compared to patients within the same co-morbidity group who were boosted, including:
- People with diabetes (2.7 times)
- Patients with hypertension (2.8 times)
- Patients with chronic pulmonary disease (2.9 times)
- Patients with cancer (2.5 times)
- Patients who are immunocompromised (2.1 times)
More information about these results and comprehensive definitions of fully vaccinated is available in the research blog.
Transparency and reproducibility: The first published clinical study from Truveta
When I reflect on the industry challenges we are trying to tackle at Truveta – privacy, fragmentation, unstructured data – building trust in the data is critical. In November 2021, our then 3-person team shared the first insights with a clinical study on COVID-19 and co-morbidities. They built and published this study in just three weeks. In January 2022, the CDC validated that work by issuing a study with remarkably similar results for the same time period – but two months later.
Recently, our Truveta Research team easily reproduced the study with updated data. Today, I was excited to share how anyone in our Truveta community can reproduce the study themselves. All the data upon which the Truveta clinical study was conducted is available in the platform. All the code behind the Truveta study is available open source on GitHub. It is also available as a pre-print on MedRxiv. Truveta members can easily reproduce, comment, and build upon our study. The study is under peer review at a reputable medical journal.
The CDC study has no similar transparency. We can’t reproduce or inspect the study. It is opaque.
Representation, transparency, and reproducibility will build trust in medical research, the likes of which we sorely needed two years ago at the onset of the pandemic and are critical to the learning health system we need every day to treat patients for every condition. As an industry, we need to learn faster and have trust in the data.
An invitation
Today is another major step forward in realizing our vision of saving lives. With each new health system that joins Truveta and contributes their data, we can research rarer conditions and deliver better care. We can have a real impact on the world by bringing healthcare data together for the good of all people. We invite more health systems to join us on this journey, and if you’re interested in learning more about the Truveta platform, we’d love to hear from you.
Follow us on LinkedIn. We will be continuing to publish our progress in building our team, industry partnerships, and ongoing insights from the Truveta platform on our feed.
— Terry
