Authors: Brianna M. Goodwin Cartwright, MS ⊕Truveta, Inc, Bellevue, WA, Patricia J. Rodriguez, PhD, MPH ⊕Truveta, Inc, Bellevue, WA, Duy Do, PhD ⊕Truveta, Inc, Bellevue, WA, Nicholas Stucky, MD, PhD ⊕Truveta, Inc, Bellevue, WA
- Side effects were the most common reported reason for AOM and ADM GLP-1 RA discontinuation (28.2%).
- AOM discontinuation was high throughout 2023 and early 2024 due to medication unavailability.
This blog is an extension of our poster presented at ISPOR 2025, titled Real-world temporal and indication-specific variation in drivers of GLP-1 RA discontinuation.
In recent years, there has been a significant increase in the number of patients being prescribed glucagon-like peptide-1 receptor agonists (GLP-1 RAs), a class of medications used to manage both type 2 diabetes and obesity (1–4). Our previous work has shown that GLP-1 RAs make up 5% of prescriptions within the US (although this trend has increased, as seen in the latest GLP-1 prescribing and dispense report). These medications have shown promising results in helping patients improve glycemic control and achieve weight loss, making them a popular treatment option (5–9).
However, our prior work has shown that many patients stop taking GLP-1 RAs prematurely. Specifically, 46.5% of patients with type 2 diabetes and 64.8% without diabetes discontinue within one year (10). The difference for people with and without diabetes suggests that the reasons patients stop treatment may be complex and influenced by multiple factors.
Despite the growing use of these medications, there is still limited understanding of why patients are unable to continue them or choose to discontinue them. Even less is known about how these reasons differ between those using GLP-1 RAs as anti-obesity medications (AOMs) versus those using them for antidiabetic management (ADMs), or how these patterns have changed over time as awareness, access, and prescribing practices have evolved.
Our ISPOR poster aimed to address this gap. Specifically, we aimed to examine the reasons for GLP-1 RA discontinuation across different time periods and to investigate whether these reasons differ depending on the medication’s intended use—whether prescribed for weight loss or for diabetes management. This study aims to add to the literature to inform future strategies to improve long-term adherence and support patients in reaching their treatment goals. You can view the full code and data definitions used in Truveta Studio.
Methods
Using a subset of Truveta Data, we identified patients who had a prescription for a GLP-1 RA between June 2021 and December 2024. Patients were required to have evidence of a GLP-1 RA prescription fill and documented reason for discontinuation from a GLP-1 RA. These reasons were extracted from clinical notes.
Patients were excluded if they had a prescription fill within 60 days after the date of discontinuation or if the GLP-1 RA indication (AOM or ADM) could not be determined.
GLP-1 RA medications have different approved indications; the indications have different doses of the same drug. For example, AOM medications, like Wegovy (AOM semaglutide) or Zepbound (AOM tirzepatide), are approved for weight loss, while ADM medications like Ozempic (ADM semaglutide) and Mounjaro (ADM tirzepatide), are approved for treatment of type 2 diabetes. In this study, the brand name was extracted from the discontinuation note and used to determine if the drug was an AOM or ADM. If these data were unavailable, the brand name of the most recent GLP-1 RA prescription fill was used to infer the indication. In cases in which multiple discontinuation events were recorded, only the most recent event was analyzed.
Group differences in discontinuation reasons between AOM and ADM users were assessed using chi-squared tests. Temporal trends in discontinuation reasons were described across the study period. AOM trends were plotted starting in April 2023 to allow time for medications to become available after FDA approval, people to initiate, and discontinue.
Results
Patient population
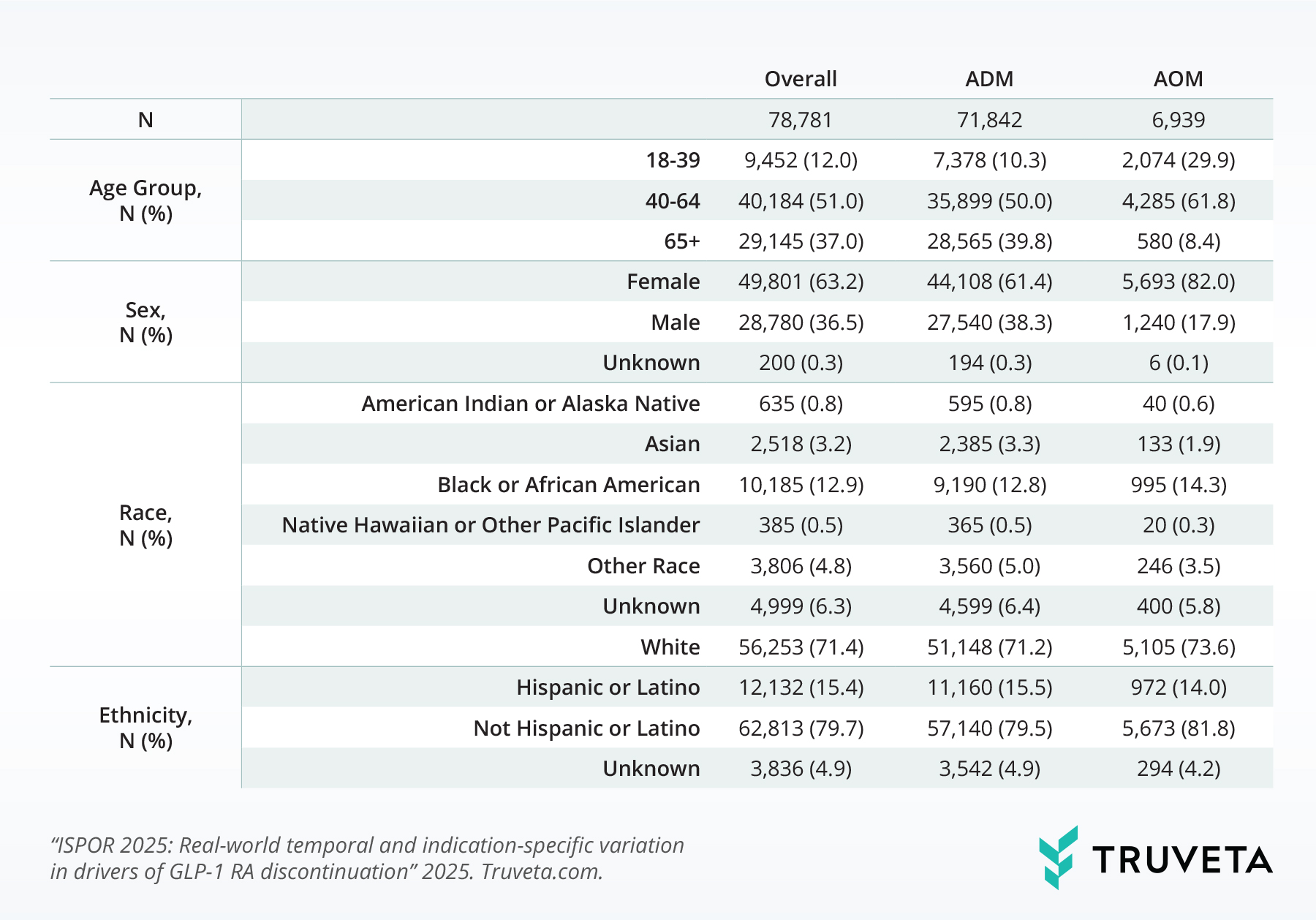
We included 78,781 patients who had both a prescription for a GLP-1 receptor agonist (GLP-1 RA) and a documented reason for discontinuing the medication between June 2021 and December 2024 (Table 1). The vast majority—more than 91%—were using these medications for diabetes management (ADM), rather than for weight loss (AOM).
Table 1: Patient demographics
Reasons for discontinuation
Across the entire population, side effects were the leading reason patients stopped taking GLP-1 RAs, accounting for 28.2% of all discontinuations (Table 2). Importantly, a substantial number of patients had multiple reasons noted for stopping (21%), underscoring the complexity of real-world medication use and the challenges patients face when using these therapies. Cost was also a notable barrier (12.8% of patients discontinued due to affordability).
Table 2: Distribution of reasons for GLP-1 RA discontinuation by indication
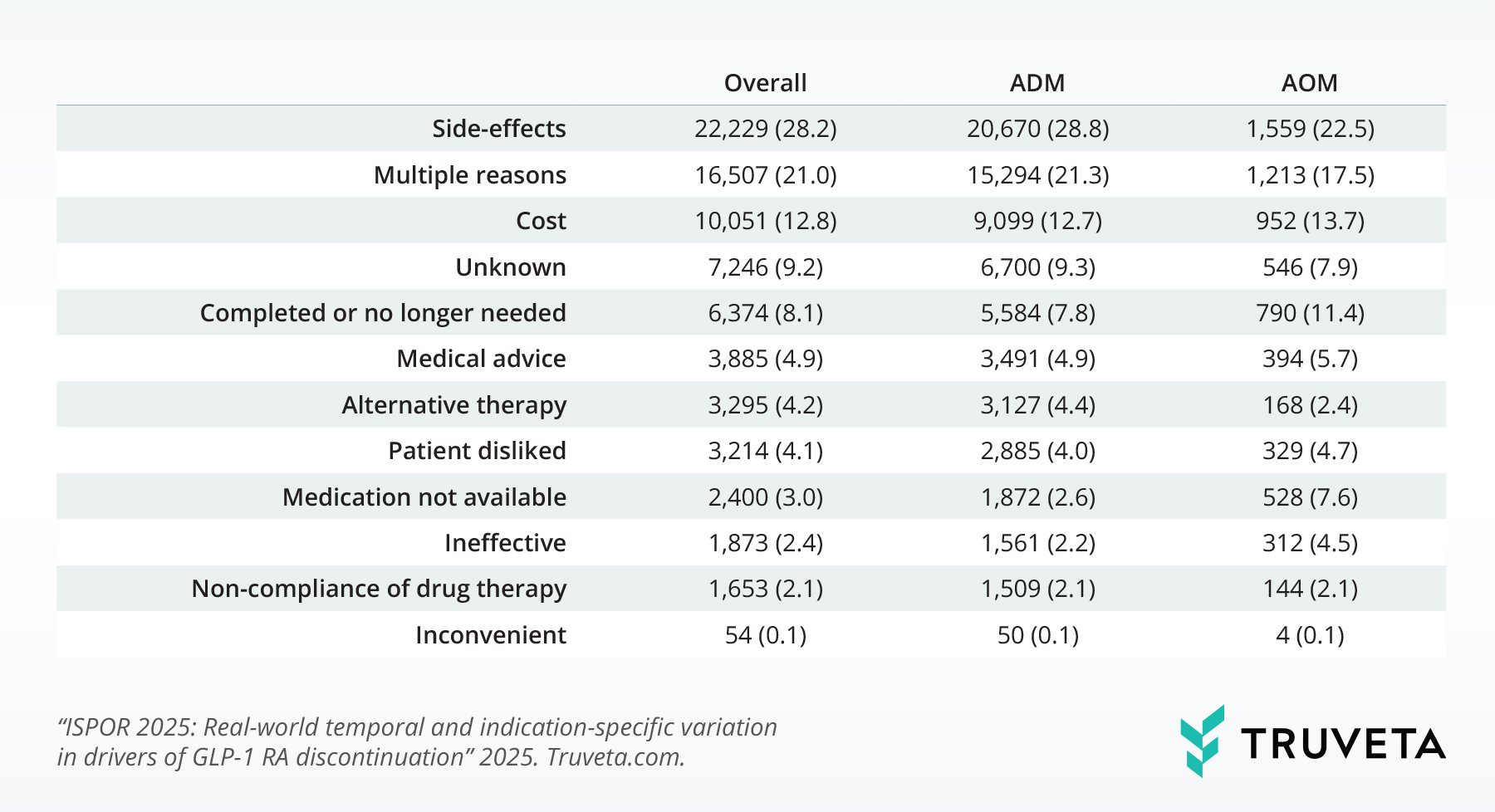
We observed significant differences in the reasons for discontinuation between patients using GLP-1 RAs for diabetes versus those using them for weight management (p < 0.001), suggesting that the patient experience varies depending on the medication’s intended use.
Trends in discontinuation by indication
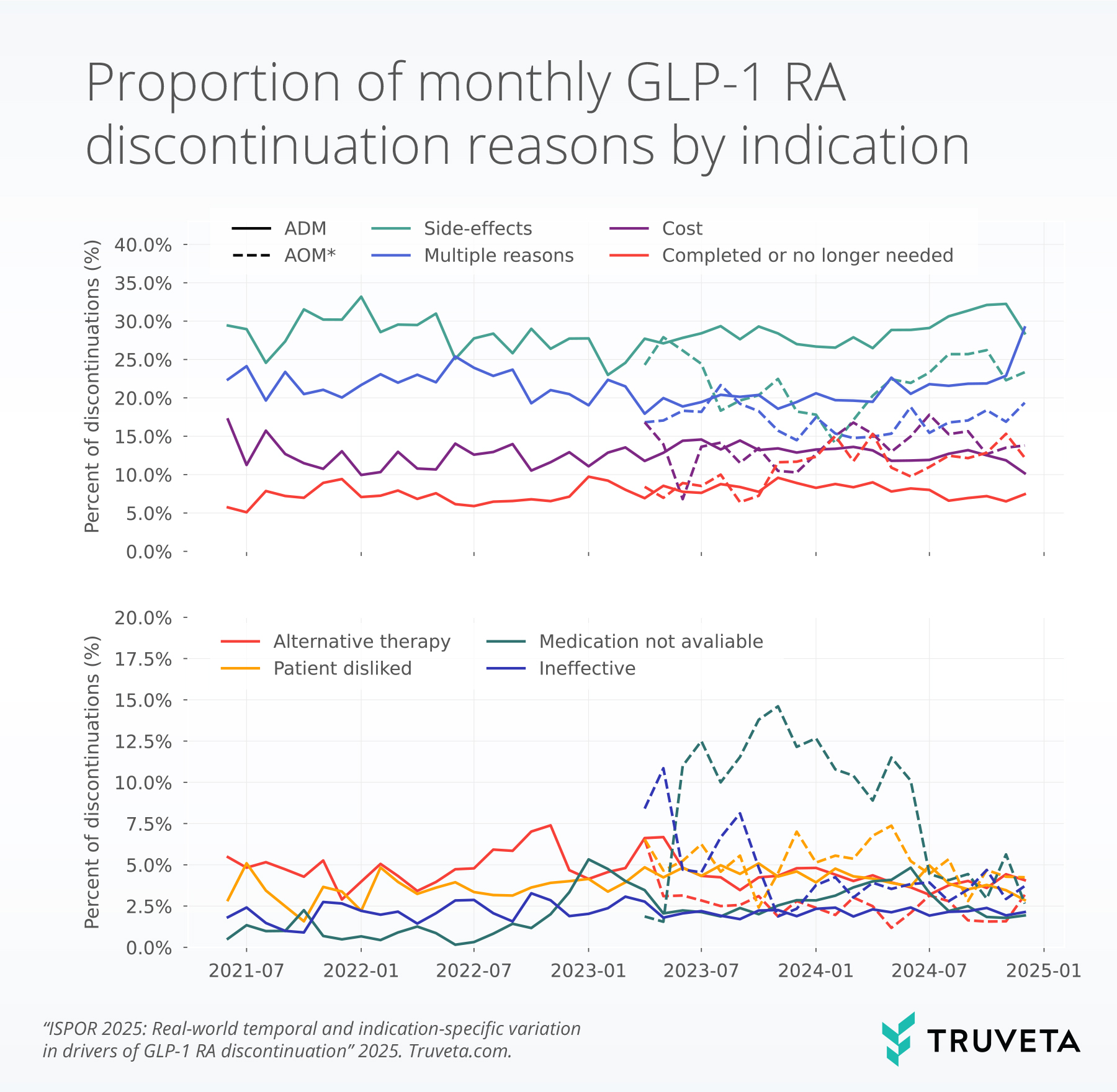
Side effects were consistently the top reason for stopping treatment for patients receiving ADM and AOM GLP-1s.
Medication availability became a more prominent issue during specific time periods; patients receiving ADMs saw noticeable spikes in the rate of discontinuation due to medication availability in January 2023 (5.3%) and June 2024 (4.8%), while patients receiving AOMs experienced higher rates of discontinuation due to medication availability throughout 2023 and into early 2024. In November 2023, the rate of discontinuations due to medication availability peaked at 14.6%.
Discontinuation due to therapy completion or feeling that the medication was no longer needed was more common in the AOM discontinuation group compared to the ADM group.
Discussion
As the use of GLP-1 RAs continues to rise in the US for both diabetes and obesity management (1, 2), understanding why patients discontinue these therapies is critical for improving long-term treatment outcomes. Our findings build on previous research showing high discontinuation rates, particularly among individuals using GLP-1 RAs for weight management (10), and provide new insights into how and why these medications are stopped in real-world clinical settings.
Consistent with earlier studies, side effects emerged as the most commonly cited reason for discontinuation, regardless of medication indication. Although we did not look at sex differences in discontinuation reasons, research work has shown that women experience nausea and vomiting (side effects) at 2.5-fold higher rates than men (11). This highlights the ongoing need for patient education and provider support around managing tolerability, particularly during dose escalation phases. In this study, a large portion of patients reported multiple discontinuation reasons, reflecting the multifaceted challenges involved in GLP-1 RA use — including tolerability, cost, access, and individual treatment goals.
We also found that cost remains a substantial barrier to sustained use, with nearly 13% of patients citing affordability as a reason for stopping. Prior reports have also cited cost to be a barrier to use for a large portion of patients, with monthly costs up to $1349 before insurance, rebates, or other discounts (2, 12). This is particularly relevant as public demand for these medications grows and payers navigate coverage policies for both anti-obesity and anti-diabetic indications. Ensuring equitable access will be essential if these therapies are to reach their full potential in improving population health.
Patients using GLP-1 RAs for weight loss (AOM) were more likely to discontinue due to perceived completion of therapy or no longer needing the medication — suggesting that some view these drugs as short-term solutions rather than components of ongoing chronic disease management. In contrast, those using the medications for diabetes (ADM) showed more stable discontinuation patterns, though they still faced challenges related to side effects and medication availability.
The timing of supply-related discontinuations further illustrates how external factors, like national shortages or surges in demand, can abruptly disrupt continuity of care (13–15). In this study, we saw disruptions were particularly pronounced among AOM users, peaking in late 2023. This reflects broader market dynamics following FDA approvals and may be influenced by increasing off-label use.
This study has multiple limitations. First, we did not require patients to continuously take a GLP-1 RA for a pre-specified amount of time before discontinuation. However, we did require that patients had a prescription fill of the GLP-1 RA and had a reason for discontinuation. Second, a large percentage of patients had multiple reasons for discontinuation in their notes. We did not investigate the overlap in these reasons; however, this highlights the complexity of discontinuation from this class of drugs. Finally, previous research has shown there may be sex differences in the rate of people who experience side effects, however we did not explore differences in discontinuation reasons by sex. More research is needed to understand the complex relationship between sex and discontinuation reasons.
These results underscore the importance of personalized care strategies and proactive follow-up to ensure patients remain on therapy when appropriate—or transition off it safely when needed. As more people initiate GLP-1 RAs for diverse indications, understanding these nuanced experiences will be key to improving adherence, minimizing harm, and optimizing outcomes.
These findings are consistent with data accessed on April 24, 2025.
Citations
- S. Gratzl, B. M. G. Cartwright, P. J. Rodriguez, K. Gilbert, D. Do, N. Masters, N. L. Stucky, Monitoring Report: GLP-1 RA Prescribing Trends – March 2025 Data. [Preprint] (2025). https://doi.org/10.1101/2025.03.06.25323524.
- A. Montero, G. Sparks, M. Presiado, L. Hamel, KFF Health Tracking Poll May 2024: The Public’s Use and Views of GLP-1 Drugs. Kaiser Family Foundation (2024).
- U.S. Food & Drug Administration, “FDA approves first oral GLP-1 treatment for type 2 diabetes” (2019); https://www.fda.gov/news-events/press-announcements/fda-approves-first-oral-glp-1-treatment-type-2-diabetes.
- U.S. Food & Drug Administration, “FDA Approves New Medication for Chronic Weight Management” (2023); https://www.fda.gov/news-events/press-announcements/fda-approves-new-medication-chronic-weight-management.
- A. J. Ahmann, M. Capehorn, G. Charpentier, F. Dotta, E. Henkel, I. Lingvay, A. G. Holst, M. P. Annett, V. R. Aroda, Efficacy and Safety of Once-Weekly Semaglutide Versus Exenatide ER in Subjects With Type 2 Diabetes (SUSTAIN 3): A 56-Week, Open-Label, Randomized Clinical Trial. Diabetes Care 41, 258–266 (2018).
- W. T. Garvey, J. P. Frias, A. M. Jastreboff, C. W. Le Roux, N. Sattar, D. Aizenberg, H. Mao, S. Zhang, N. N. Ahmad, M. C. Bunck, I. Benabbad, X. M. Zhang, F. H. Abalos, F. C. P. Manghi, C. J. Zaidman, M. L. Vico, D. Aizenberg, P. R. Costanzo, L. P. Serra, I. J. MacKinnon, M. N. Hissa, M. H. Vidotti, J. F. Kerr Saraiva, B. B. Alves, D. R. Franco, O. Moratto, S. Murthy, G. Goyal, Y. Yamasaki, N. Sato, S. Inoue, T. Asakura, M. Shestakova, E. Khaykina, E. Troshina, N. Vorokhobina, A. Ametov, S.-T. Tu, C.-Y. Yang, I.-T. Lee, C.-N. Huang, H.-Y. Ou, G. Freeman, S. Machineni, K. Klein, S. Sultan, A. Parsa, J. Otero-Martinez, A. Gonzalez, A. Bhargava, S. Brian, C. Ince, S. Plantholt, J. Cole, A. Lacour, D. Vega, J. De Souza, J. L. Rohlf, R. C. St. John, B. Horowitz, H. Audish, R. Galindo, G. Umpiperrez, J. Ard, B. Curtis, W. T. Garvey, N. J. Fraser, J. Mandry, R. Mohseni, R. Mayfield, T. Powell, C. Vance, S. Ong, A. L. Lewy-Alterbaum, A. Murray, A. Al-Karadsheh, T. Yacoub, K. Roberts, D. L. Fried, J. Rosenstock, B. Pulla, B. Bode, J. Frias, L. Klaff, R. Brazg, J. Van, A. Tan, T. Briskin, M. Rhee, T. Chaicha-Brom, P. A. Hartley, L. Nunez, G. Cortes-Maisonet, G. Soucie, S. Hsia, T. Jones, Tirzepatide once weekly for the treatment of obesity in people with type 2 diabetes (SURMOUNT-2): a double-blind, randomised, multicentre, placebo-controlled, phase 3 trial. The Lancet 402, 613–626 (2023).
- R. E. Pratley, V. R. Aroda, I. Lingvay, J. Lüdemann, C. Andreassen, A. Navarria, A. Viljoen, Semaglutide versus dulaglutide once weekly in patients with type 2 diabetes (SUSTAIN 7): a randomised, open-label, phase 3b trial. The Lancet Diabetes & Endocrinology 6, 275–286 (2018).
- J. P. H. Wilding, R. L. Batterham, S. Calanna, M. Davies, L. F. Van Gaal, I. Lingvay, B. M. McGowan, J. Rosenstock, M. T. D. Tran, T. A. Wadden, S. Wharton, K. Yokote, N. Zeuthen, R. F. Kushner, Once-Weekly Semaglutide in Adults with Overweight or Obesity. N Engl J Med 384, 989–1002 (2021).
- D. Rubino, N. Abrahamsson, M. Davies, D. Hesse, F. L. Greenway, C. Jensen, I. Lingvay, O. Mosenzon, J. Rosenstock, M. A. Rubio, G. Rudofsky, S. Tadayon, T. A. Wadden, D. Dicker, STEP 4 Investigators, M. Friberg, A. Sjödin, D. Dicker, G. Segal, O. Mosenzon, M. Sabbah, Y. Sofer, V. Vishlitzky, E. W. Meesters, M. Serlie, A. Van Bon, H. Cardoso, P. Freitas, P. Carneiro De Melo, M. Monteiro, M. Monteiro, D. Rodrigues, A. Badat, P. Joshi, G. Latiff, E. A. Mitha, H. H. Snyman, E. Van Nieuwenhuizen, O. González Albarrán, A. Caixas, C. De Al Cuesta, P. P. Garcia Luna, C. Morales Portillo, P. Mezquita Raya, M. A. Rubio, N. Abrahamsson, J. Hoffstedt, F. Von Wowern, E. Uddman, B. Bach-Kliegel, F. Beuschlein, S. Bilz, A. Golay, G. Rudofsky, C. Strey, G. Fadieienko, N. Kosei, T. Tatarchuk, V. Velychko, O. Zinych, S. L. Aronoff, H. E. Bays, A. P. Brockmyre, R. S. Call, C. Crump, C. V. Desouza, V. Espinosa, A. L. Free, W. H. Gandy, S. A. Geller, G. M. Gottschlich, F. L. Greenway, L. Han-Conrad, W. Harper, L. Herman, M. Hewitt, P. Hollander, S. R. Kaster, A. Manessis, F. A. Martin, R. E. McNeill, A. V. Murray, P. C. Norwood, J. C. H. Reed, J. Rosenstock, D. M. Rubino, M. J. Schear, M. L. Warren, Effect of Continued Weekly Subcutaneous Semaglutide vs Placebo on Weight Loss Maintenance in Adults With Overweight or Obesity: The STEP 4 Randomized Clinical Trial. JAMA 325, 1414 (2021).
- P. J. Rodriguez, V. Zhang, S. Gratzl, D. Do, B. Goodwin Cartwright, C. Baker, T. J. Gluckman, N. Stucky, E. J. Emanuel, Discontinuation and Reinitiation of Dual-Labeled GLP-1 Receptor Agonists Among US Adults With Overweight or Obesity. JAMA Network Open 8, e2457349–e2457349 (2025).
- T. Roseberry, I. Grossrubatscher, T. Krausz, Y. Wang, M. Schwartz, D. Tingley, Sex differences in GLP-1 signaling across species. [Preprint] (2025). https://doi.org/10.1101/2025.03.17.643822.
- H. Klein, Most Insured Adults Still Have to Pay at Least Part of the Cost of GLP-1 Drugs (2024). https://www.ajmc.com/view/most-insured-adults-still-have-to-pay-at-least-part-of-the-cost-of-glp-1-drugs.
- U.S. Food & Drug Administration, Current and Resolved Drug Shortages and Discontinuations Reported to FDA (2025). https://dps.fda.gov/drugshortages/resolved/semaglutide-injection.
- H. P. Whitley, J. M. Trujillo, J. J. Neumiller, Special Report: Potential Strategies for Addressing GLP-1 and Dual GLP-1/GIP Receptor Agonist Shortages. Clinical Diabetes 41, 467–473 (2023).
- U.S. Food & Drug Administration, Current and Resolved Drug Shortages and Discontinuations Reported to FDA (2025). https://dps.fda.gov/drugshortages/resolved/tirzepatide-injection.