- Although a majority of women report intrauterine device (IUD) insertion to be moderately to severely painful, so far in 2025, only 1 in 20 women received pain medication on the day of IUD insertion.
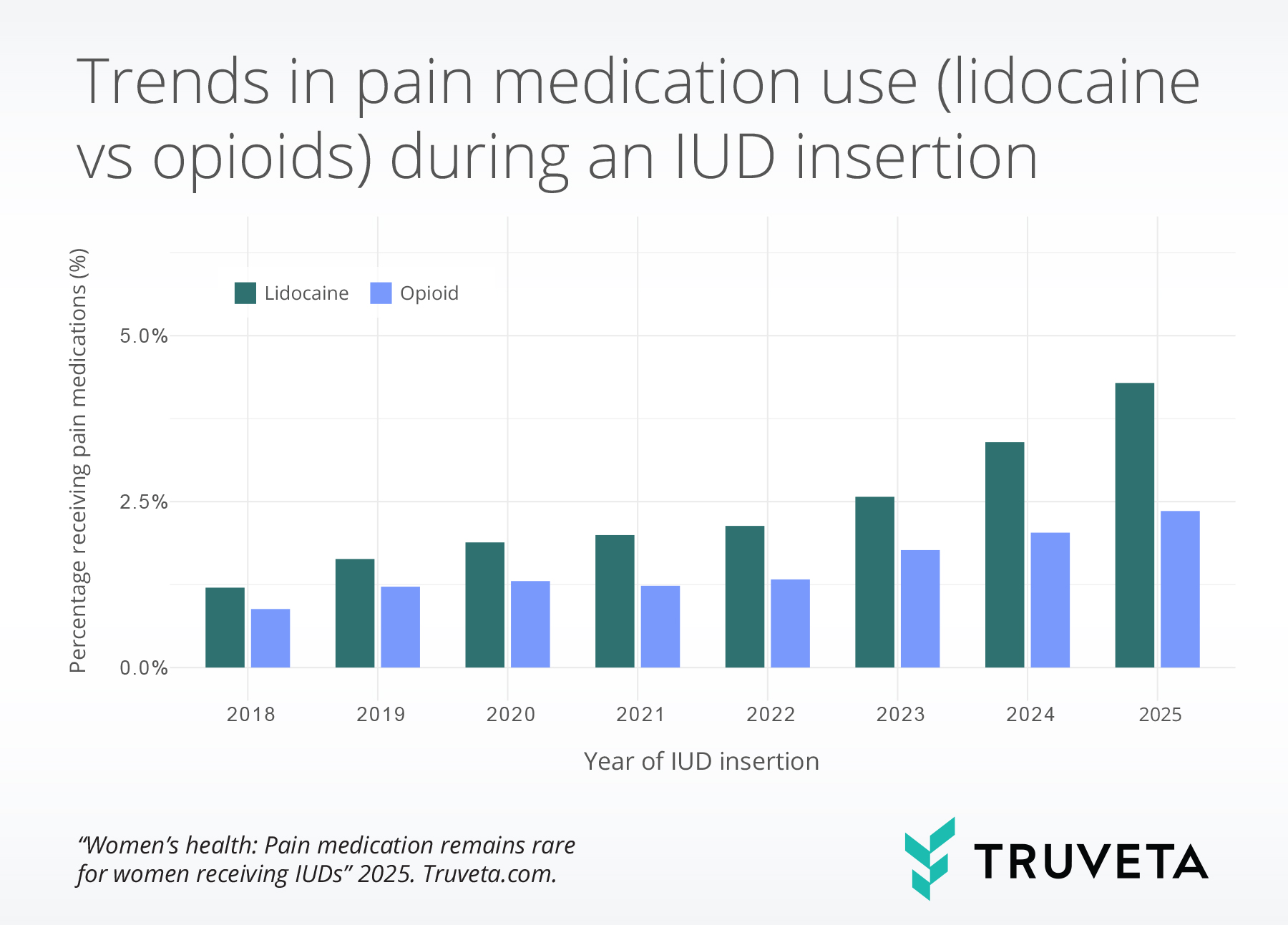
- The use of pain medication during IUD insertions more than doubled between 2018 and 2025, driven largely by an increased use of lidocaine.
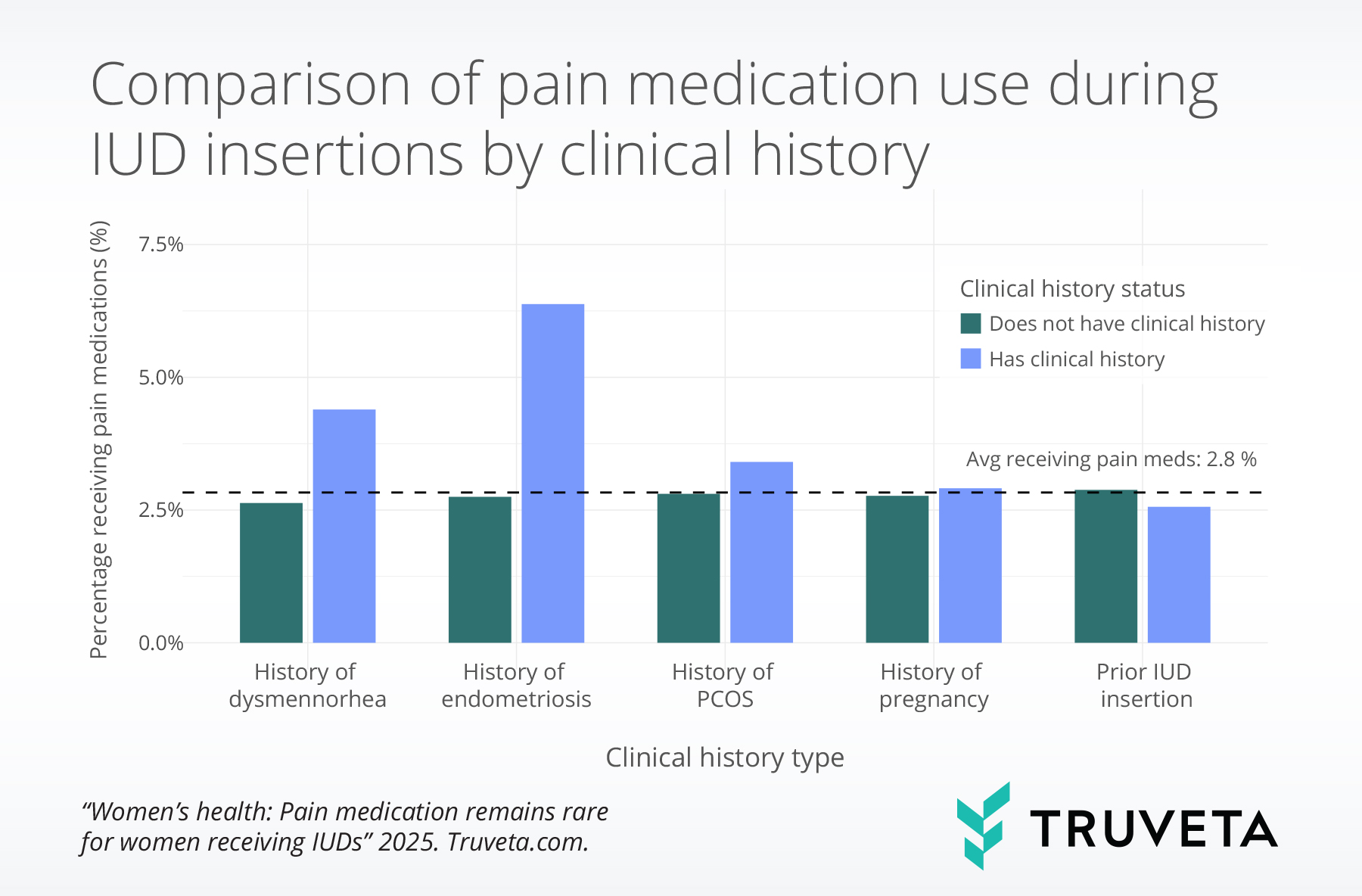
- Pain medication during IUD insertions was 2.3 times more common among patients with endometriosis, 1.2 times more common for patients with polycystic ovary syndrome (PCOS), and 1.7 times more common for patients with dysmenorrhea compared to patients without those conditions.
Intrauterine devices (IUDs) are highly effective forms of reversible contraception, with the ability to prevent pregnancy for up to 3 to 10 years (1, 2). IUDs are also used for non-contraceptive benefits, such as reducing heavy periods, relieving painful cramps, and reducing pelvic pain associated with endometriosis (3).
Despite their effectiveness, IUD insertion is known to be painful for many patients (4–6). Studies show that a significant majority of women — ranging between 57-100% — report the insertion as moderately or severely painful (7–11). Factors such as no prior pregnancy, a history of dysmenorrhea (a condition characterized by painful menstrual periods caused by uterine contractions), and patient anxiety are all associated with higher levels of pain (5, 8, 12–16).
Nonsteroidal anti-inflammatory drugs (NSAIDs), such as ibuprofen, are commonly recommended to patients prior to IUD insertion to reduce cramping and discomfort (17). However, evidence supporting their effectiveness is limited. Multiple randomized trials and systematic reviews have shown that NSAIDs do not significantly reduce IUD insertion-related pain, suggesting other forms of pain management, such as lidocaine, may be more effective (18–20).
Lidocaine, a local anesthetic, has shown promise in reducing pain during IUD insertion, though its effectiveness varies depending on the medication strength and form (e.g., cream, spray, injection) (21–29). In response to emerging evidence and evolving clinical practice patterns, the CDC updated its recommendations in August 2024 to include lidocaine as a preferred option for pain management during IUD insertion (30). In addition to lidocaine, opioids — particularly, tramadol — have also been studied as a potential intervention for IUD insertion pain, although they are not commonly used in this context (15, 31).
This study aims to describe real-world trends in the use of pain medications during IUD insertion over time, with a particular focus on lidocaine and opioids. We evaluate how pain management practices have evolved in recent years and explore differences in use by patient characteristics such as age, race, and clinical history (such as prior pregnancies, comorbidities, and prior IUD insertion). Understanding these trends may help inform future clinical guidelines and highlight opportunities to improve comfort and access for individuals undergoing IUD insertions.
Methods
We analyzed a subset of Truveta Data that included female patients aged 15–49 years who had an IUD insertion between January 1, 2018 and March 31, 2025. We identified patients who received either lidocaine or an opioid medication on the same day as their IUD insertion and calculated the proportion of patients who received pain medications. The rates were calculated independently; therefore, patients who received both lidocaine and an opioid were counted in both analyses.
We excluded patients who underwent other gynecological procedures that might require pain medication on the same day as the IUD insertion, such as cervical biopsy or hysteroscopy. We also excluded patients who had received an IUD within the preceding three months or had received pain medication in the month prior to the IUD insertion. These exclusions were intended to remove cases where pain medication use could be related to another treatment or ongoing condition, rather than the IUD insertion itself. We identified the procedures using CPT and SNOMED codes. You can view the data definitions used to identify these codes in the study directly in Truveta Studio.
To assess how pain medication use might differ based on clinical history, we also identified patients with a prior IUD (three months or more before the current insertion), a history of pregnancy, or diagnoses of dysmenorrhea, endometriosis, or polycystic ovary syndrome (PCOS).
Results
We identified 308,327 IUD insertions between January 2018 and March 2025. The population was 72.1% White, 10.1% Black or African American (henceforth referred to as Black), and 4.9% Asian. The majority of the population was aged 25–34 (40.3%), followed by 35–49 (34.7%), and 15–24 (25.1%). Across the study period, 2.8% of patients received either lidocaine or opioids on the date of the IUD insertion.
Increase in pain medications over time
The overall rate of pain medication increased from 1.2% in 2018 to 5.2% in 2025, reflecting a 203.7% increase. So far in in 2025 only 1 in 20 women who received an IUD received pain medication. Lidocaine use saw the most substantial increase, rising from 1.2% in 2018 to 4.3% in 2025—an increase of 256.4%. Opioid use also rose, though to a lesser extent, from 0.9% in 2018 to 2.4% in 2025—an increase of 167.6%.
Among patients who received pain medication, lidocaine use increased from 70.6% in 2018 to 83.2% in 2025.
Clinical history
Patients with a history of dysmenorrhea were 1.7 times more likely to receive pain medication, those with a history of endometriosis were 2.3 times more likely, and those with a history of PCOS were 1.2 times more likely than those without these conditions.
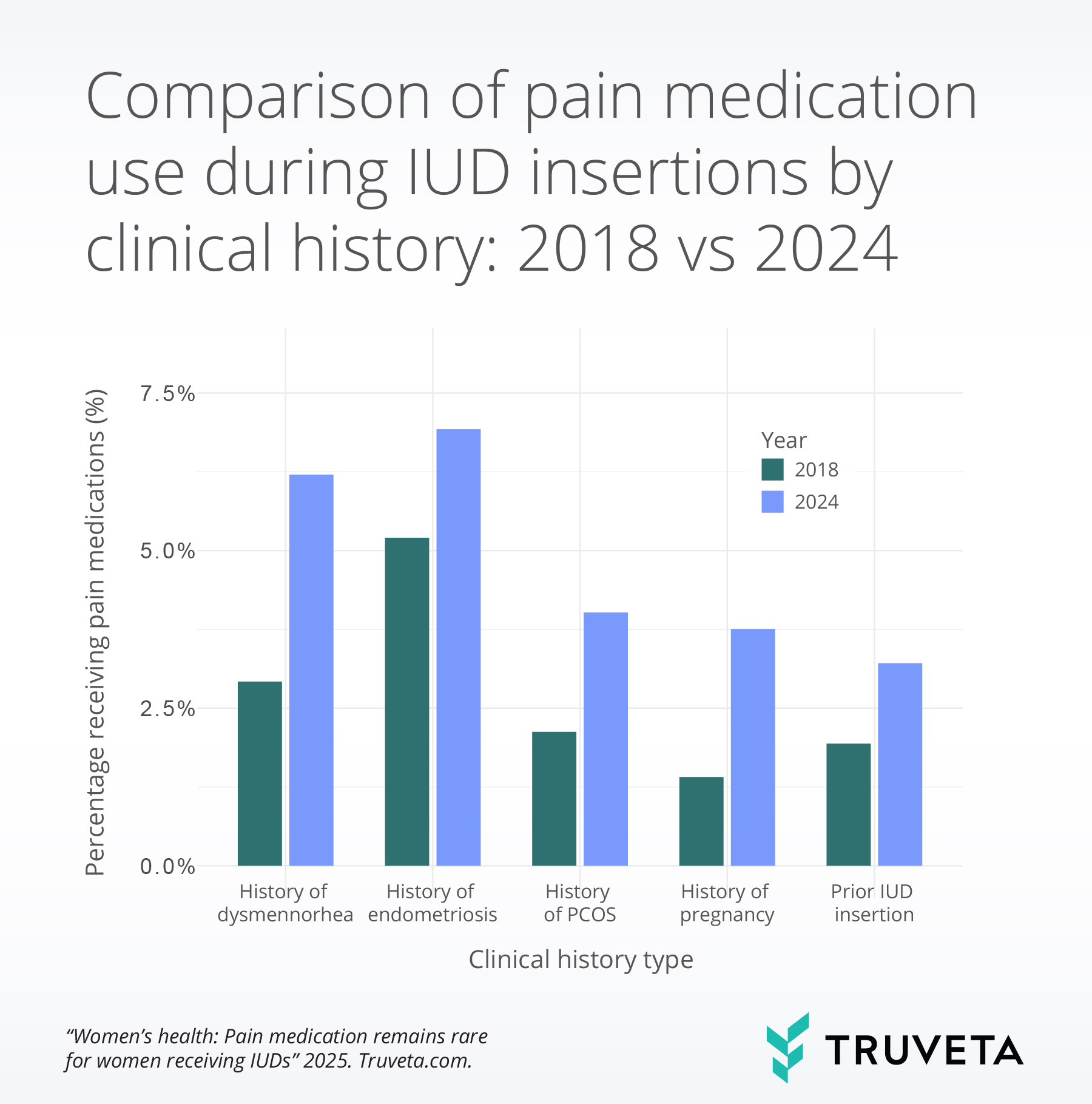
Between 2018 and 2024, the percentage of patients who received pain medications during IUD insertions increased across all clinical history and condition groups.
Among patients with dysmenorrhea, the rate more than doubled, jumping from 2.8% to 6.2%. Those with endometriosis and PCOS also showed meaningful increases, with rates rising from 5.5% to 6.8% and 2.1% to 4.0%, respectively.
Individuals with a history of IUD use experienced a rise from 1.9% to 3.3%, and those with a history of pregnancy saw their rates nearly triple—from 1.4% to 3.8%—highlighting a consistent upward trend across diverse patient populations.
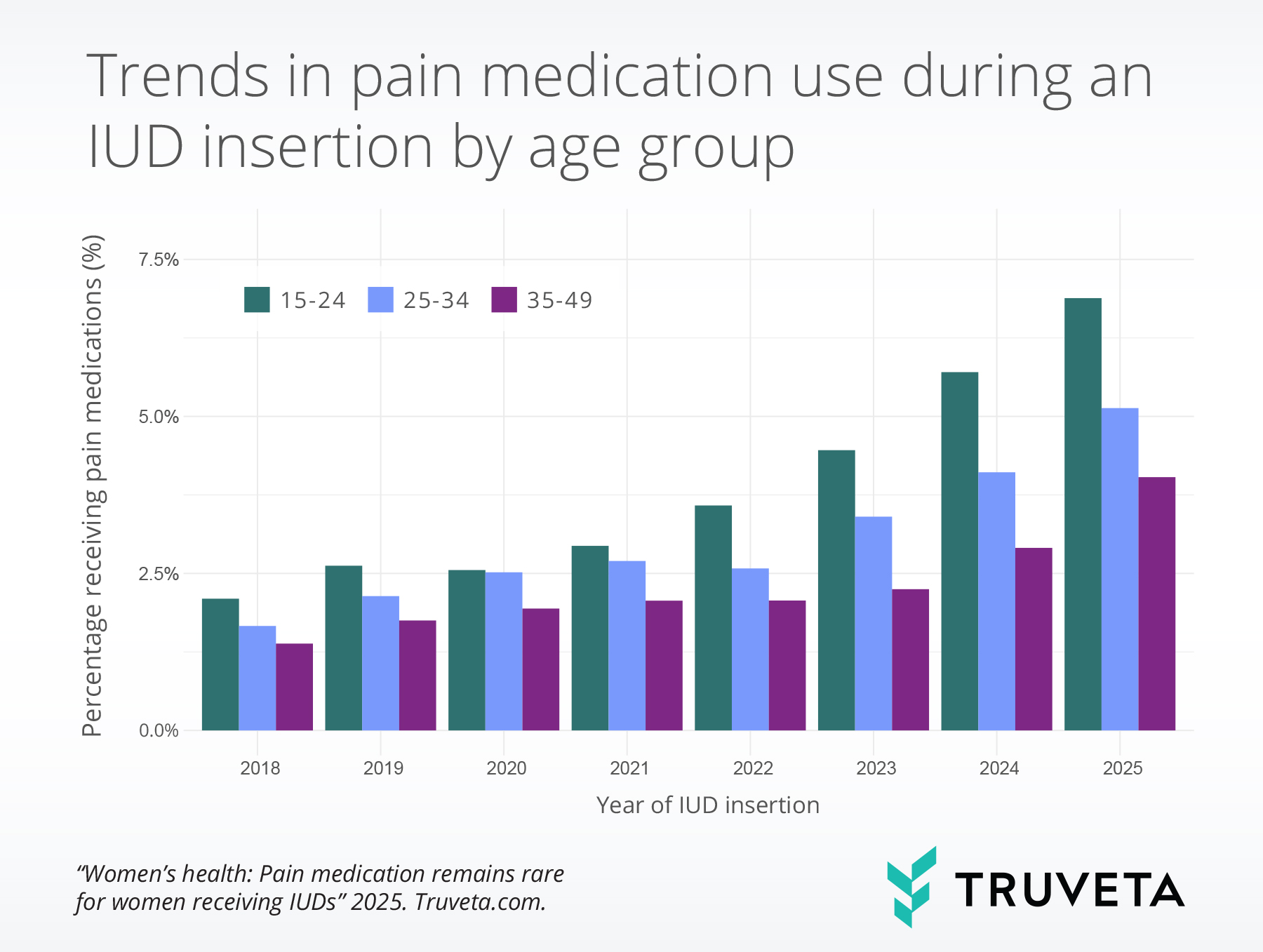
Age
All age groups experienced a substantial increase in pain medication use during IUD insertions from 2018 to 2025, though the degree of increase varied.
Among patients aged 15-25, usage rose from 2.1% to 6.9% (+228.2%), among those aged 25–34, usage rose from 1.7% to 5.1% (+208.5%), and among patients aged 35–49, usage rose from 1.4% to 4.0% (+191.6%).
Discussion
In this study, we observed a substantial increase in the use of pain medications—particularly lidocaine—during IUD insertions. Although overall usage remained low, the rate of pain medication more than doubled over the study period. These trends align with broader shifts in clinical practice, including the CDC’s August 2024 recommendation endorsing lidocaine as a preferred option for pain management during IUD insertion.
Our findings are consistent with those from a recent retrospective study conducted within the VA medical system. The study also observed an increase in pain medication use during IUD insertions from 2018 to 2023 across 28,717 insertions (17). Our study included over 10x the number of IUD insertions (308,327); this larger sample allows for a more robust and generalizable view of pain management practices across a wider range of healthcare systems and patient populations. Additionally, the VA study’s focus on a veteran population may not fully reflect the broader, more diverse demographics of the general US population, making our findings especially relevant for a wider array of patients.
We also found that pain medication use varied by clinical history. Patients with conditions associated with increased pelvic pain—such as dysmenorrhea, endometriosis, and PCOS—were more likely to receive pain medication during IUD insertion.
Several studies have found that patients with dysmenorrhea are more likely to experience pain during IUD insertion (10, 15, 32). Mechanistically, conditions like endometriosis, PCOS, and dysmenorrhea may involve chronic inflammation, scarring, or structural changes in the pelvis that increase sensitivity and make IUD insertions more painful (33–36). In this report, we found higher rates of pain medication for patients with these conditions; clinicians may be more attuned to the needs of patients with these conditions, given the link between these conditions and higher procedural pain (10, 15, 32). Further, patients with these conditions may also have more frequent interactions with the health system, which could make them more likely to advocate for pain relief based on prior experiences (37, 38).
We also observed variation in pain medication use by age. While all age groups experienced an increase from 2018 to 2025, younger patients (ages 15–25) saw the largest change. This trend may reflect greater advocacy from younger patients, generational shifts in expectations around pain management, and age-related differences in pregnancy history.
This study has several limitations. First, the IUD insertions included in this study were performed within a Truveta member healthcare system; these do not represent all the places people receive IUD insertions, such as standalone family planning clinics, Planned Parenthood, and community health centers. Additionally, we did not assess the timing, dosage, or route of administration for medications, which may influence effectiveness and patient experience. Further research is needed to understand the effectiveness of pain medications during IUD insertion.
This study contributes to the limited body of real-world data on pain management during IUD insertion. Our findings suggest pain medication use is increasing, though overall levels remain low. In 2025, only about 1 in 20 women who got an IUD received pain medication despite a majority of women considering IUD insertion to be moderately to severely painful (7–11).
These are preliminary research findings and not peer reviewed. Data are constantly changing and updating. These findings are consistent with data accessed on April 18, 2025.
Citations
- P. F. Thonneau, T. E. Almont, Contraceptive efficacy of intrauterine devices. American Journal of Obstetrics and Gynecology 198, 248–253 (2008).
- J. Trussell, Contraceptive failure in the United States. Contraception 83, 397–404 (2011).
- J. Yoost, Understanding benefits and addressing misperceptions and barriers to intrauterine device access among populations in the United States. Patient Prefer Adherence 8, 947–957 (2014).
- A. Brockmeyer, Kishen ,Meera, A. and Webb, Experience of IUD/IUS insertions and clinical performance in nulliparous women–a pilot study. The European Journal of Contraception & Reproductive Health Care 13, 248–254 (2008).
- T. Hunter, S. Sonalkar, C. Schreiber, L. Perriera, M. Sammel, A. Akers, Anticipated Pain During Intrauterine Device Insertion. J Pediatr Adolesc Gynecol 33, 27–32 (2020).
- D. G. Callahan, L. F. Garabedian, K. F. Harney, A. D. DiVasta, Will it Hurt? The Intrauterine Device Insertion Experience and Long-Term Acceptability Among Adolescents and Young Women. Journal of Pediatric and Adolescent Gynecology 32, 615–621 (2019).
- L. Marions, Lövkvist ,Lena, Taube ,Annika, Johansson ,Monica, Dalvik ,Håkan, I. and Øverlie, Use of the levonorgestrel releasing-intrauterine system in nulliparous women – a non-interventional study in Sweden. The European Journal of Contraception & Reproductive Health Care 16, 126–134 (2011).
- J. Kaislasuo, O. Heikinheimo, P. Lähteenmäki, S. Suhonen, Predicting painful or difficult intrauterine device insertion in nulligravid women. Obstetrics & Gynecology 124, 345–353 (2014).
- H. Akintomide, N. Brima, R. D. E. Sewell, J. M. Stephenson, Patients’ experiences and providers’ observations on pain during intrauterine device insertion. The European Journal of Contraception & Reproductive Health Care 20, 319–326 (2015).
- L. S. Ferreira, M. N. de Nadai, O. B. Poli-Neto, S. A. Franceschini, C. R. Juliato, I. M. U. Monteiro, L. Bahamondes, C. S. Vieira, Predictors of severe pain during insertion of the levonorgestrel 52 mg intrauterine system among nulligravid women. Contraception 102, 267–269 (2020).
- E. A. Lopes-Garcia, Carmona ,Elenice V., Monteiro ,Ilza, L. and Bahamondes, Assessment of pain and ease of intrauterine device placement according to type of device, parity, and mode of delivery. The European Journal of Contraception & Reproductive Health Care 28, 163–167 (2023).
- D. Hubacher, V. Reyes, S. Lillo, A. Zepeda, P.-L. Chen, H. Croxatto, Pain from copper intrauterine device insertion: Randomized trial of prophylactic ibuprofen. American Journal of Obstetrics and Gynecology 195, 1272–1277 (2006).
- N. Brima, H. Akintomide, V. Iguyovwe, S. Mann, A comparison of the expected and actual pain experienced by women during insertion of an intrauterine contraceptive device. OAJC, 21 (2015).
- B. Dina, L. J. Peipert, Q. Zhao, J. F. Peipert, Anticipated pain as a predictor of discomfort with intrauterine device placement. American journal of obstetrics and gynecology 218, 236-e1 (2018).
- K. Gemzell-Danielsson, D. Mansour, C. Fiala, A. M. Kaunitz, L. Bahamondes, Management of pain associated with the insertion of intrauterine contraceptives. Human reproduction update 19, 419–427 (2013).
- Y. Akdemir, M. Karadeniz, The relationship between pain at IUD insertion and negative perceptions, anxiety and previous mode of delivery. The European Journal of Contraception & Reproductive Health Care 24, 240–245 (2019).
- A. D. Ware, T. L. Blumke, P. J. Hoover, Z. P. Veigulis, J. M. Ferguson, M. Pillai, T. F. Osborne, National assessment on the frequency of pain medication prescribed for intrauterine device insertion procedures within the Veterans Affairs Health Care System. PloS one 20, e0308427 (2025).
- P. H. Bednarek, M. D. Creinin, M. F. Reeves, C. Cwiak, E. Espey, J. T. Jensen, Prophylactic ibuprofen does not improve pain with IUD insertion: a randomized trial. Contraception 91, 193–197 (2015).
- I. Martingano, E. Lakey, D. Raskin, K. Rowland, Efficacy of NSAIDs in reducing pain during intrauterine device Insertion: A systematic review. European Journal of Obstetrics & Gynecology and Reproductive Biology 309, 219–225 (2025).
- R. H. Allen, D. Bartz, D. A. Grimes, D. Hubacher, P. O’Brien, Interventions for pain with intrauterine device insertion. Cochrane Database of Systematic Reviews (2009).
- A. Y. Akers, C. Steinway, S. Sonalkar, L. K. Perriera, C. Schreiber, J. Harding, J. F. Garcia-Espana, Reducing Pain During Intrauterine Device Insertion: A Randomized Controlled Trial in Adolescents and Young Women. Obstetrics & Gynecology 130, 795 (2017).
- A. Samy, A. M. Abbas, M. Mahmoud, A. Taher, M. H. Awad, T. El husseiny, M. Hussein, M. Ramadan, M. A. Shalaby, M. El sharkawy, D. Hatem, A. Alaa-El-din Wali, S. M. Abd-el-fatah, A. H. Hussein, H. Haggag, Evaluating different pain lowering medications during intrauterine device insertion: a systematic review and network meta-analysis. Fertility and Sterility 111, 553-561.e4 (2019).
- C. Anthoulakis, E. Iordanidou, A. Vatopoulou, Pain perception during levonorgestrel-releasing intrauterine device insertion in nulliparous women: a systematic review. Journal of Pediatric and Adolescent Gynecology 31, 549–556 (2018).
- H. Aksoy, Ü. Aksoy, S. Ozyurt, G. Açmaz, M. Babayigit, Lidocaine 10% spray to the cervix reduces pain during intrauterine device insertion: a double-blind randomised controlled trial. Journal of Family Planning and Reproductive Health Care 42, 83–87 (2016).
- F. R. Perez-Lopez, Martinez-Dominguez ,Samuel J., Perez-Roncero ,Gonzalo R., A. V. and Hernandez, Uterine or paracervical lidocaine application for pain control during intrauterine contraceptive device insertion: a meta-analysis of randomised controlled trials. The European Journal of Contraception & Reproductive Health Care 23, 207–217 (2018).
- L. M. Lopez, A. Bernholc, Y. Zeng, R. H. Allen, D. Bartz, P. A. O’Brien, D. Hubacher, Interventions for pain with intrauterine device insertion. Cochrane Database of Systematic Reviews (2015).
- S. Sandoval, M. E. Meurice, N. B. Pebley, S. K. Mody, Alleviating Pain with IUD Placement: Recent Studies and Clinical Insight. Curr Obstet Gynecol Rep 11, 12–20 (2022).
- L. B. Zapata, T. C. Jatlaoui, P. A. Marchbanks, K. M. Curtis, Medications to ease intrauterine device insertion: a systematic review. Contraception 94, 739–759 (2016).
- A. M. Abbas, M. S. Abdellah, M. Khalaf, M. Bahloul, N. H. Abdellah, M. K. Ali, A. M. Abdelmagied, Effect of cervical lidocaine–prilocaine cream on pain perception during copper T380A intrauterine device insertion among parous women: A randomized double-blind controlled trial. Contraception 95, 251–256 (2017).
- K. M. Curtis, U.S. Selected Practice Recommendations for Contraceptive Use, 2024. MMWR Recomm Rep 73 (2024).
- S. Karabayirli, A. A. Ayrım, B. Muslu, Comparison of the Analgesic Effects of Oral Tramadol and Naproxen Sodium on Pain Relief During IUD Insertion. Journal of Minimally Invasive Gynecology 19, 581–584 (2012).
- R. Schneyer, K. Lerma, J. Conti, K. Shaw, Dysmenorrhoea as a risk factor for pain with intrauterine device insertion. BMJ Sex Reprod Health 48, e31–e37 (2022).
- K. C. Schliep, S. L. Mumford, C. M. Peterson, Z. Chen, E. B. Johnstone, H. T. Sharp, J. B. Stanford, A. O. Hammoud, L. Sun, G. M. Buck Louis, Pain typology and incident endometriosis. Hum. Reprod. 30, 2427–2438 (2015).
- E. E. Kamal, H. A. Hamada, R. S. Ashour, A. M. Yousef, R. M. Elbesh, Biomechanical changes in females with poly cystic ovarian syndrome: a case–control study. Scientific Reports 15, 11190 (2025).
- J. Maddern, L. Grundy, J. Castro, S. M. Brierley, Pain in endometriosis. Frontiers in cellular neuroscience 14, 590823 (2020).
- L. D. Ireland, R. H. Allen, Pain management for gynecologic procedures in the office. Obstetrical & gynecological survey 71, 89–98 (2016).
- L. Mikesell, A. C. Bontempo, Healthcare Providers’ Impact on the Care Experiences of Patients with Endometriosis: The Value of Trust. Health Communication 38, 1981–1993 (2023).
- M. Ismayilova, S. Yaya, “I felt like she didn’t take me seriously”: a multi-methods study examining patient satisfaction and experiences with polycystic ovary syndrome (PCOS) in Canada. BMC Women’s Health 22, 47 (2022).

