- The proportion of patients initiating treatment for multiple sclerosis (MS) with a high efficacy disease-modifying therapy (DMT) more than doubled from 2018 to 2025, reflecting evolving clinical guidelines.
- Use of oral and injectable therapies showed a gradual decline as infusion treatments gained a modest increase in initiation rates.
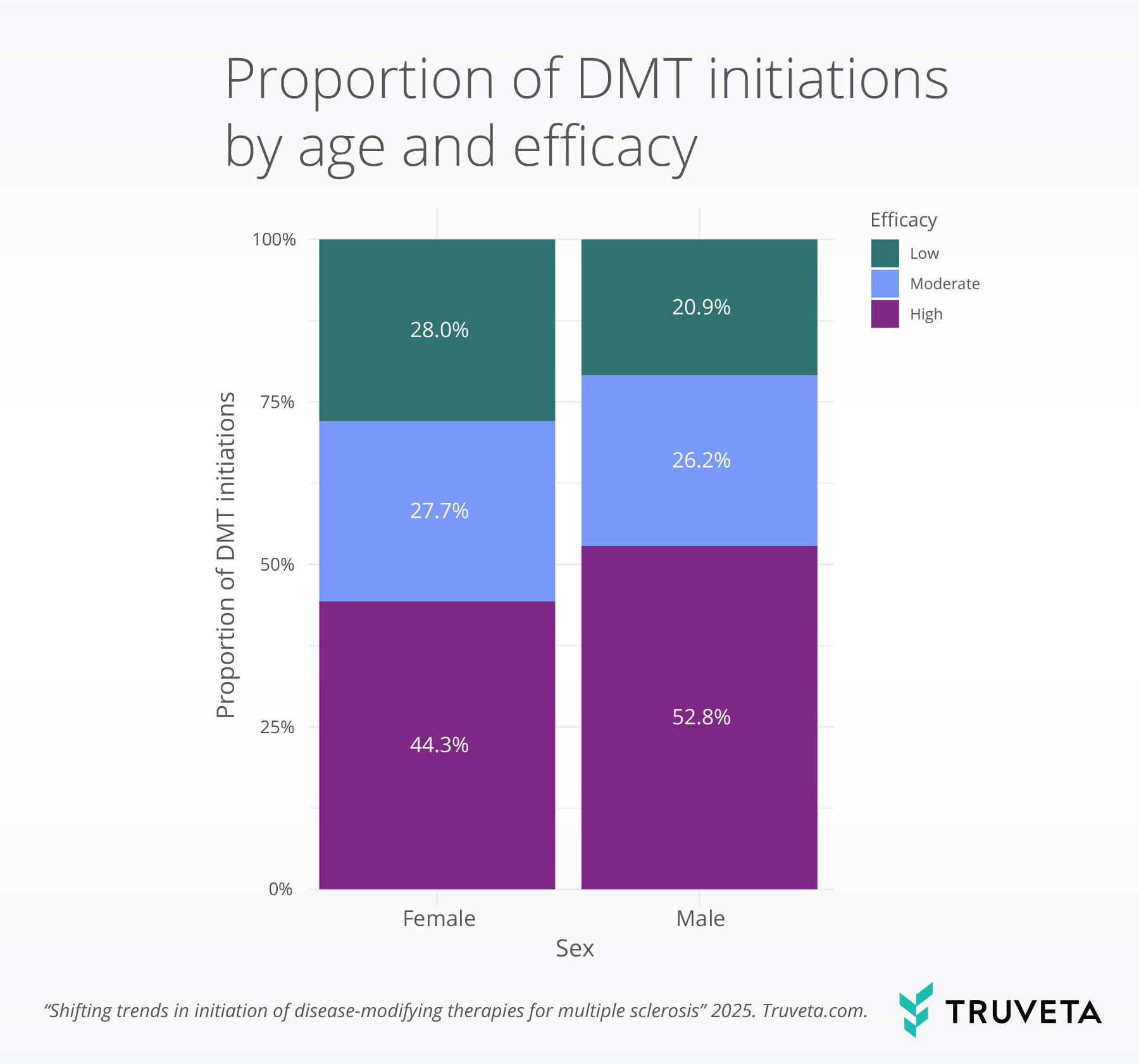
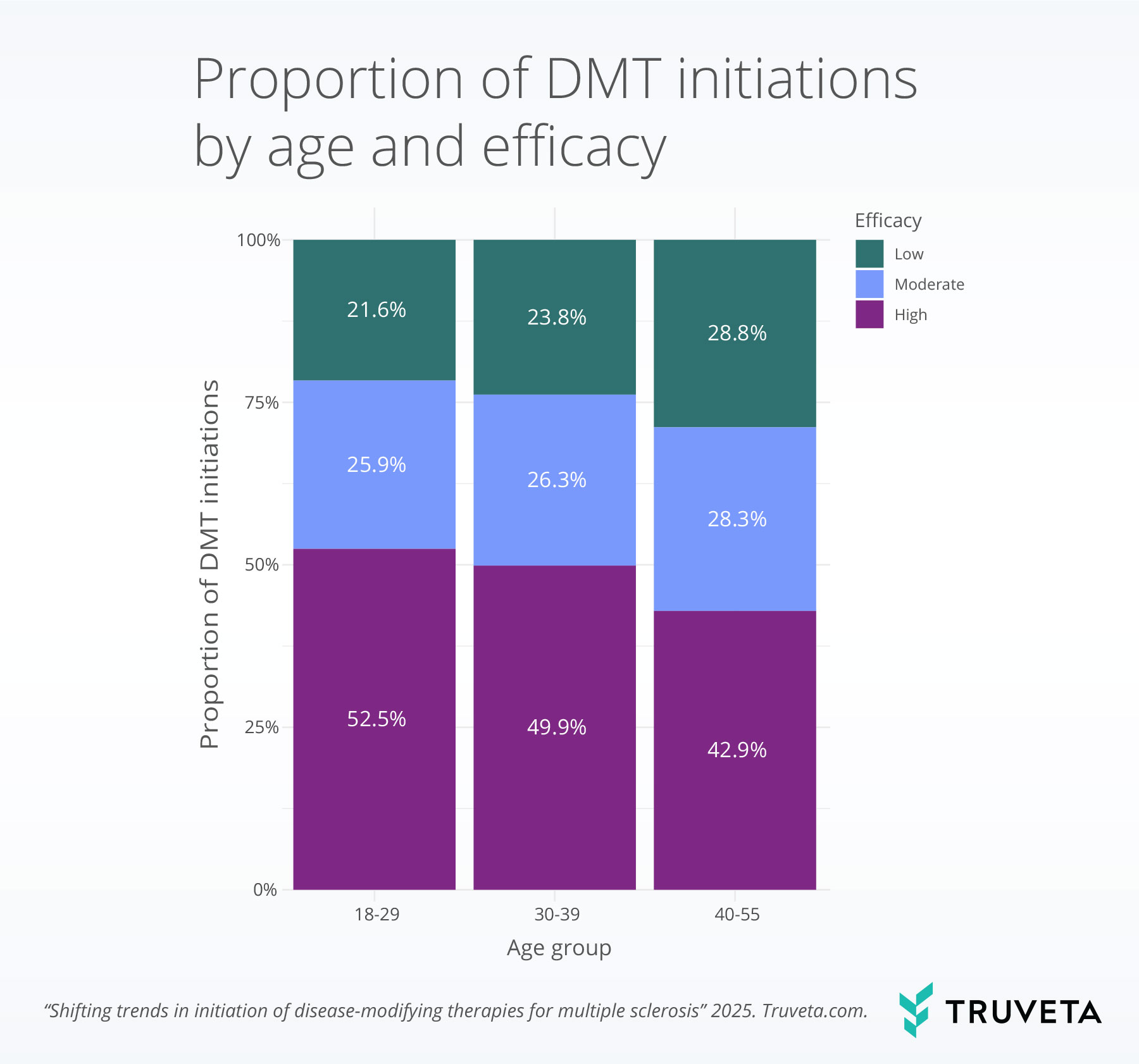
- Male patients and younger adults were more likely to start high-efficacy therapies, highlighting demographic differences in treatment patterns.
Multiple sclerosis (MS) is a disease that affects the nervous system by damaging the coating that protects nerve fibers, known as the myelin sheath (1, 2). Patients with MS can experience symptoms such as fatigue, vision disturbances, and trouble walking; these symptoms often worsen over time and can greatly reduce quality of life (3, 4).
While there’s no cure for MS, disease-modifying therapies (DMTs) can slow disease progression, reduce relapses, and help preserve function—making early treatment essential (1, 2, 5). Historically, MS treatment used an escalation strategy, where patients initiated medications that had fewer risks and less impact, and only transitioned to stronger or more effective therapies if the disease worsened. (6–8). While patients with MS might be treated with high-, moderate-, or low-efficacy DMTs, recent research has shown that starting with high-efficacy DMTs early in the disease course may lead to better long-term outcomes, including reducing long-term disability (9–12). The American Academy of Neurology (AAN) updated its guidelines in 2018 to support earlier use of high-efficacy therapies in patients with relapsing multiple sclerosis who have poor prognostic indicators (13). Building on this, the Multiple Sclerosis Treatment Consensus Group (MSTCG) released a paper in 2021 advocating for the early use of high-efficacy DMTs, emphasizing that early intervention offers the best chance to alter the disease course and prevent long-term disability (14).
Since 2018, the number of approved disease-modifying therapies (DMTs) for multiple sclerosis has grown rapidly, offering patients more options across all three main routes of administration: oral, injectable, and infused. Notable recent approvals include oral medications such as siponimod (2019), ozanimod (2020), and ponesimod (2021); injectable medications like ofatumumab (2020); and infused medications like ublituximab (2022) (15, 16).
Following the approval of oral disease-modifying therapies (DMTs) in 2010, utilization of these treatments has risen, accompanied by a corresponding decrease in the use of injectable options. (16–19). This shift highlights how the introduction of new therapies can reshape the treatment landscape and influence patient choices. Route of administration plays a central role in these decisions, affecting not only convenience but also patient preferences, access, and adherence—making it a critical factor in treatment selection.
Building on this evolving treatment landscape, our study aimed to understand real-world trends in DMT initiation since 2018. We examined initiation patterns by both efficacy level and route of administration. Understanding these patterns is critical for evaluating the real-world adoption of new therapies and informing future treatment guidelines.
You can read this full study and explore the data definitions and code directly in Truveta Studio.
Methods
We used a subset of Truveta Data to identify adults aged 18-55 with MS who initiated a DMT, defined as having a DMT prescribed, dispensed, or administered between January 2018 and June 2025.
Patients were included if they had at least two distinct MS diagnoses and no prior DMT use, including prescriptions, dispenses, or administrations (20). To confirm these were patients regularly receiving care within a Truveta member health system, patients were also required to have outpatient encounters during two periods—0 to 6 months and 7 to 12 months prior to DMT initiation.
Patients who were pregnant within nine months before or three months after starting treatment were excluded, as these therapies are contraindicated or not considered safe during pregnancy.
Additionally, since some DMTs are approved for other conditions, we excluded patients with Crohn’s disease or chronic lymphocytic leukemia (CLL) to focus on MS-specific treatment.
DMTs
We included 19 DMTs in this analysis. DMT efficacy was classified based on a recent meta-analysis (21).
Analysis
For our analysis, we examined patterns of DMT initiation over time; results were stratified by both efficacy and route of administration. To explore drug-specific trends, we focused on the five DMTs that exhibited the most substantial proportional shifts in overall DMT use during the study period.
In addition to tracking these trends, we investigated whether DMT efficacy varied across a range of demographic and clinical factors, including sex, race, ethnicity, year, census region, Elixhauser comorbidity score, number of outpatient encounters in the year prior to DMT initiation, age, urbanicity, and marital status. We used multinomial network analysis to assess whether observed differences in DMT efficacy were linked to a particular factor, such as sex or age, once we took into account other patient characteristics.
When describing results, we use the term significant only for differences that were statistically meaningful (i.e., unlikely to have occurred by chance).
Results
We identified 6,781 patients with MS who initiated a DMT between January 2018 and June 2025. The population was 72.9% White, 22.6% Black or African American, and 0.9% Asian. The majority of the population lived in urban areas (85.7%). The majority of the population was female (78.5%), and the average age was 41.0 years at the time of DMT initiation.
Drug patterns over time
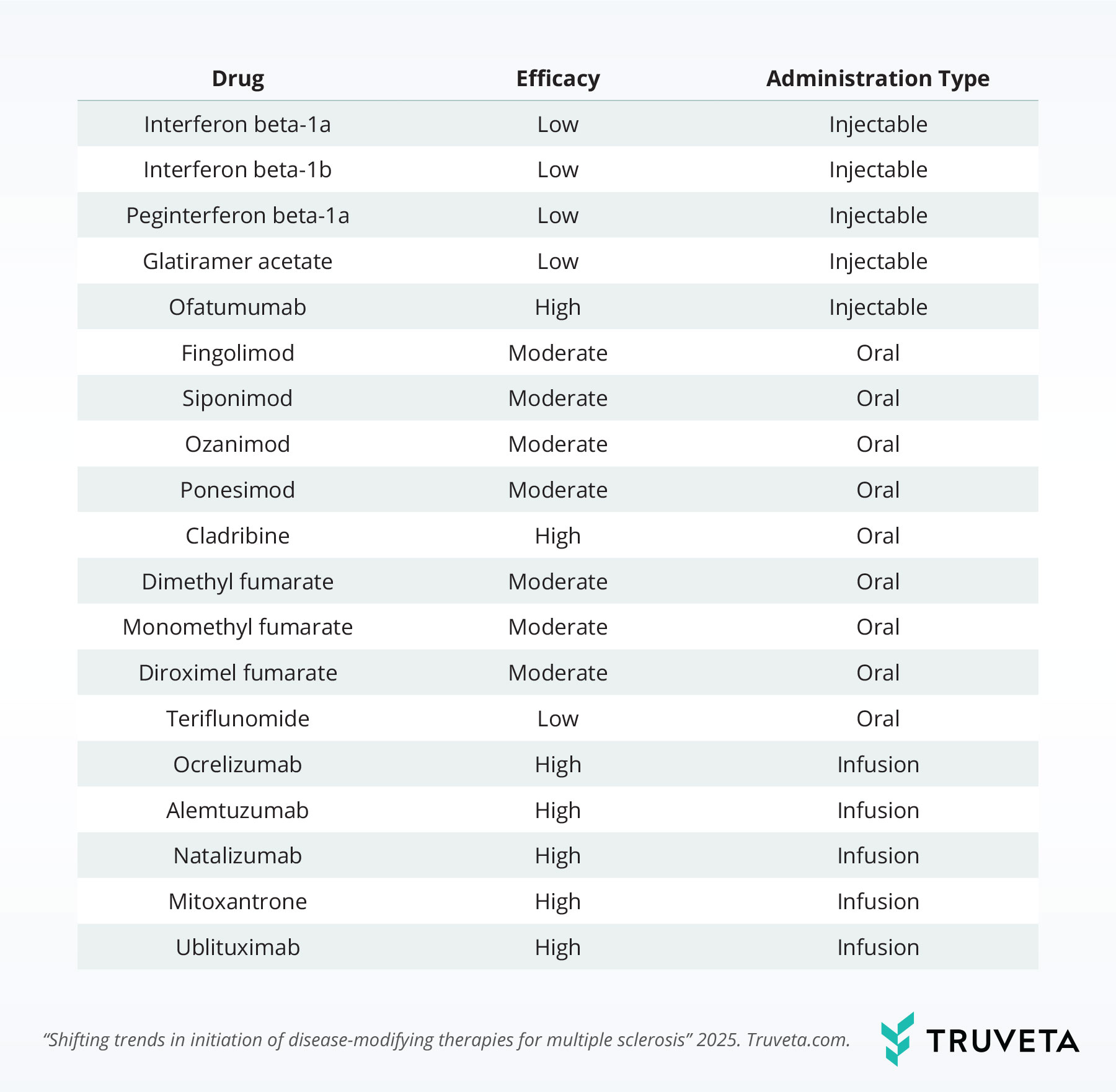
We observed notable shifts in the proportion of drugs patients initiated over time.
Dimethyl fumarate initiations declined from 17.6% in the first quarter of 2018 to 7.9% in the second quarter of 2025, a 55.1% relative decrease.
Glatiramer acetate saw a similar decline, dropping from 24.0% to 3.2%, representing an 86.8% relative decrease.
In contrast, ocrelizumab use nearly doubled, increasing from 16.6% to 37.4%, a 125.7% relative increase over the same period.
Ofatumumab also saw a notable rise, growing from 2.1% after it was first approved in August 2020 to 22.9% in the second quarter of 2025—representing an over 10-fold increase.
Lastly, the use of ublituximab—approved in December 2022—expanded threefold, rising from 1.6% to 5.0%.
Route of administration patterns over time
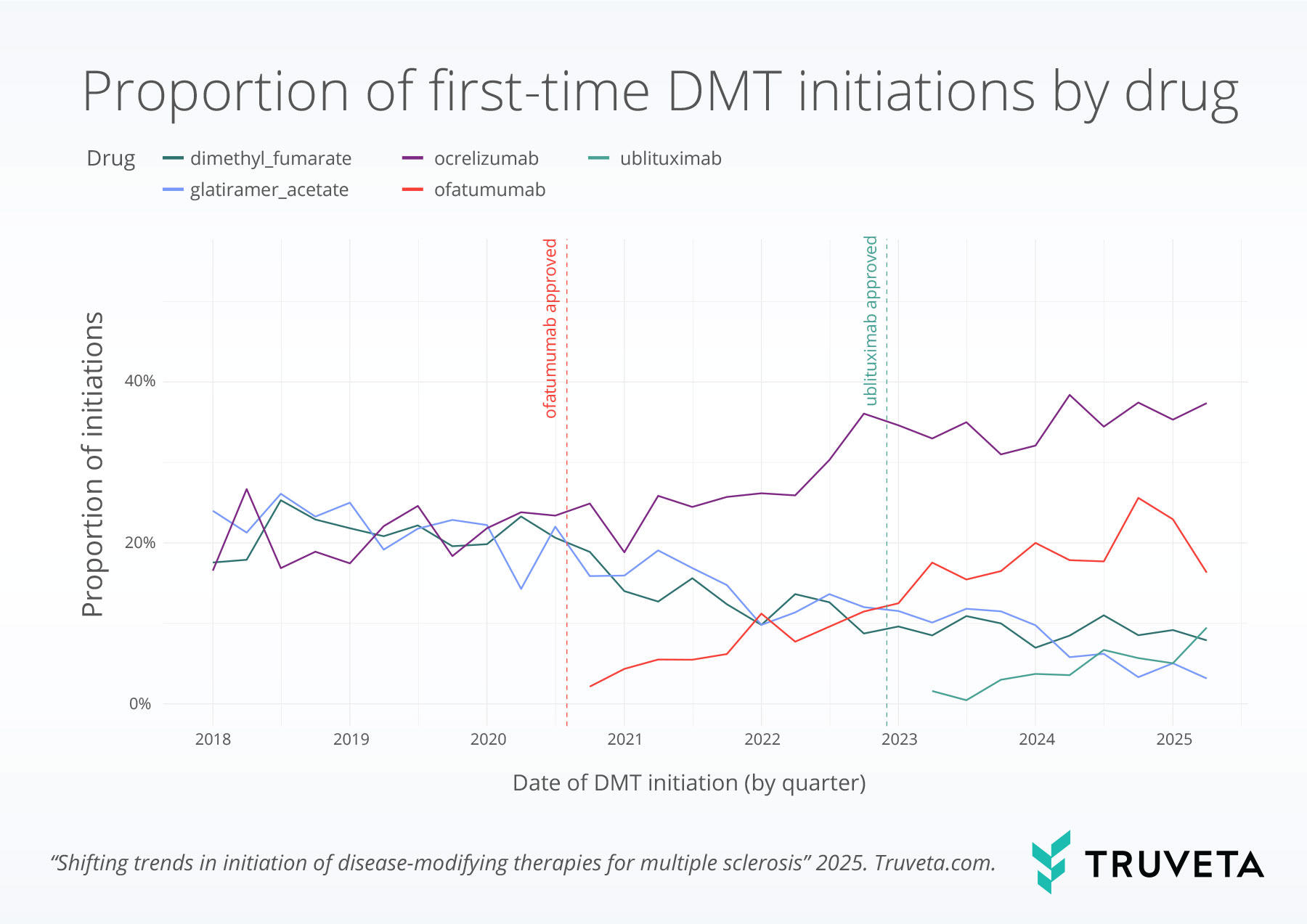
We observed clear changes in treatment delivery methods over time.
The proportion of infusion-based DMT initiations increased from 31.4% in the first quarter of 2018 to 55.3% in the second quarter of 2025—representing a 75.9% relative increase.
Meanwhile, injectable DMTs declined slightly from 34.1% to 21.1%, a 38.3% relative decrease.
Oral DMTs saw a more substantial decline, dropping from 34.5% to 23.7%, a 31.3% relative decrease over the same period.
Efficacy patterns over time
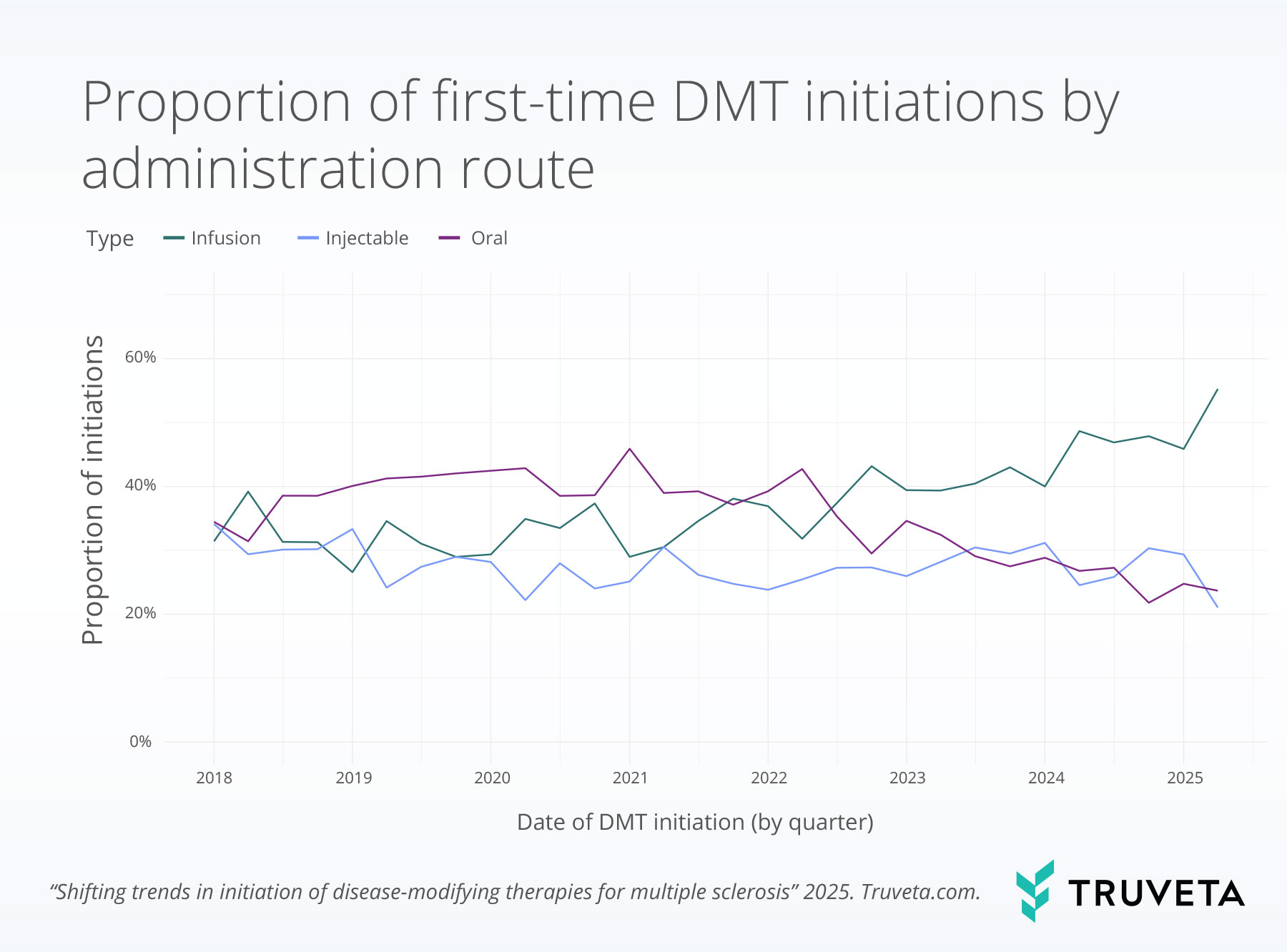
We observed a notable shift in DMT efficacy over time.
High-efficacy DMTs rose from 31.4% of all initiations in the first quarter of 2018 to 73.7% in the second quarter of 2025—a 134.5% relative increase.
In contrast, low- and moderate-efficacy DMTs declined from 39.5% and 29.1% to 6.3% and 20.0%, representing relative decreases of 84.0% and 31.2%, respectively.
AAN (2018) and MSTCG (2021) guidelines support starting high-efficacy MS treatments early.
Characteristics of high-efficacy DMTs
Male patients were significantly more likely to receive a high-efficacy DMT, with 52.8% of males initiating high-efficacy treatments compared to 44.3% of females.
Younger patients were also significantly more likely to receive a high-efficacy DMT, with 52.5% of patients aged 18–29 and 49.9% of those aged 30–39 initiating these treatments, compared to 42.9% of adults aged 40-55.
Discussion
In this study of over 6,000 patients with MS who initiated DMTs between 2018 and 2025, we observed substantial shifts in treatment patterns. Over the study period, there was a decline in the proportion of previously favored treatment options (moderate-efficacy DMT dimethyl fumarate and low-efficacy DMT glatiramer acetate), which accounted for more than one third of DMT initiations in early 2018 but less than 15% by 2025. This decline was accompanied by a notable increase in high-efficacy agents, especially ocrelizumab and the more recently approved ofatumumab.
We found that high-efficacy DMTs increased by 135% from 2018 to 2025. This growth appeared to follow the 2021 MSTCG guidelines more closely than the earlier 2018 recommendations from AAN. The AAN guidelines acknowledged that early use of high-efficacy therapies may be beneficial, especially for patients with poor prognostic indicators, but did not issue a strong recommendation (13). In contrast, the 2021 MSTCG guidelines clearly advocated for initiating treatment with high-efficacy agents for most patients (14). The stronger language, combined with a growing body of real-world evidence demonstrating better outcomes for patients starting on high-efficacy therapies, suggests that both the tone of clinical recommendations and the accumulation of supportive evidence were important drivers of practice change (7, 10, 11, 14).
The nearly doubling of ocrelizumab initiations and the 10-fold rise in ofatumumab use likely reflect providers’ adherence to evolving clinical practice guidelines, emerging evidence supporting greater efficacy and safety profiles, and growing provider and patient acceptance of infusion therapies, among others (8, 11). Ocrelizumab, the first therapy approved for primary progressive multiple sclerosis (PPMS), has demonstrated strong efficacy in both PPMS and relapsing-remitting MS (RRMS)—the latter characterized by episodic relapses (1, 22). Its durable long-term outcomes and convenient twice-yearly infusion schedule, now shortened to two hours, have likely contributed to its widespread adoption (22). Ofatumumab offers similar efficacy with the added convenience of monthly at-home subcutaneous injections (23, 24). These shifts align with earlier research showing a move toward high-efficacy therapies, a trend that appears to have accelerated in recent years (16).
Our findings align with earlier research that documented shifting treatment patterns over the past two decades. A national claims analysis covering 2001–2020 described increased use of oral DMTs in the early 2010s, followed by a moderate rise in infusion therapies after ocrelizumab’s approval in 2017 (16). While that study did not include data after 2020, our results suggest that the trend toward infusion and high-efficacy therapies has continued and even accelerated in recent years.
We also observed demographic patterns in DMT efficacy. Male and younger patients were more likely to initiate high-efficacy therapies. This aligns with prior research showing that males often experience more rapid disease progression, making early initiation of high-efficacy DMTs especially important (25, 26). Previous studies have also found that male patients tend to have a shorter interval between diagnosis and treatment initiation, consistent with our finding that they are more likely to begin with higher-efficacy options (27). In contrast, older adults were less likely to initiate high-efficacy DMTs, possibly reflecting the known decrease in treatment efficacy and increased risk of adverse events with age (28, 29). These factors may influence shared decision-making toward more conservative treatment approaches for older adults.
This study has a few limitations. First, we did not confirm multiple sclerosis diagnoses using the McDonald criteria (30); instead, we identified patients based on diagnosis codes and initiation of DMT, applying a previously validated claims-based algorithm (20). To capture treatment initiation, we only included individuals with no signs of any previous DMT use. However, as we only required one year of outpatient encounters prior to DMT initiation, it’s possible that some prior DMT use may not have been captured. Lastly, we did not account for clinical factors, such as MRI results or signs of disease activity, that often guide treatment decisions (31, 32). While our study highlights broad trends in DMT initiation, there is likely substantial variation in the clinical reasoning behind individual treatment choices and these findings should therefore be interpreted with caution.
Building on an evolving treatment landscape, our study focuses on trends in the initiation of DMTs for multiple sclerosis starting in 2018. We observed a clear shift toward increased use of high-efficacy DMTs, consistent with recommendations from the AAN and MSTCG supporting earlier use of these therapies. These insights help to monitor how clinical guidelines translate into practice and can inform future treatment strategies and policy decisions.
These are preliminary research findings and not peer reviewed. Data are constantly changing and updating. These findings are consistent with data accessed on July 3, 2025.
Citations
- R. Marcus, What Is Multiple Sclerosis? JAMA 328, 2078 (2022).
- S. L. Hauser, B. A. C. Cree, Treatment of Multiple Sclerosis: A Review. Am J Med 133, 1380-1390.e2 (2020).
- F. D. Lublin, D. A. Häring, H. Ganjgahi, A. Ocampo, F. Hatami, J. Čuklina, P. Aarden, F. Dahlke, D. L. Arnold, H. Wiendl, T. Chitnis, T. E. Nichols, B. C. Kieseier, R. A. Bermel, How patients with multiple sclerosis acquire disability. Brain 145, 3147–3161 (2022).
- C. Confavreux, S. Vukusic, T. Moreau, P. Adeleine, Relapses and Progression of Disability in Multiple Sclerosis. N Engl J Med 343, 1430–1438 (2000).
- V.-P. Stamatellos, G. Papazisis, Safety and Monitoring of the Treatment with Disease-Modifying Therapies (DMTs) for Multiple Sclerosis (MS). Current Reviews in Clinical and Experimental Pharmacology 18, 39–50 (2023).
- A. Gajofatto, M. D. Benedetti, Treatment strategies for multiple sclerosis: When to start, when to change, when to stop? World J Clin Cases 3, 545–555 (2015).
- T. Spelman, M. Magyari, F. Piehl, A. Svenningsson, P. V. Rasmussen, M. Kant, F. Sellebjerg, H. Joensen, J. Hillert, J. Lycke, Treatment Escalation vs Immediate Initiation of Highly Effective Treatment for Patients With Relapsing-Remitting Multiple Sclerosis: Data From 2 Different National Strategies. JAMA Neurology 78, 1197–1204 (2021).
- B. A. Singer, J. Feng, H. Chiong-Rivero, Early use of high-efficacy therapies in multiple sclerosis in the United States: benefits, barriers, and strategies for encouraging adoption. J Neurol 271, 3116–3130 (2024).
- K. Harding, O. Williams, M. Willis, J. Hrastelj, A. Rimmer, F. Joseph, V. Tomassini, M. Wardle, T. Pickersgill, N. Robertson, E. Tallantyre, Clinical Outcomes of Escalation vs Early Intensive Disease-Modifying Therapy in Patients With Multiple Sclerosis. JAMA Neurol 76, 536–541 (2019).
- M. Filippi, M. P. Amato, D. Centonze, P. Gallo, C. Gasperini, M. Inglese, F. Patti, C. Pozzilli, P. Preziosa, M. Trojano, Early use of high-efficacy disease‑modifying therapies makes the difference in people with multiple sclerosis: an expert opinion. J Neurol 269, 5382–5394 (2022).
- L. Freeman, E. E. Longbrake, P. K. Coyle, B. Hendin, T. Vollmer, High-Efficacy Therapies for Treatment-Naïve Individuals with Relapsing–Remitting Multiple Sclerosis. CNS Drugs 36, 1285–1299 (2022).
- M. D. Buron, T. A. Chalmer, F. Sellebjerg, I. Barzinji, D. Bech, J. R. Christensen, M. K. Christensen, V. Hansen, Z. Illes, H. B. Jensen, M. Kant, V. Papp, T. Petersen, S. Prakash, P. V. Rasmussen, J. Schäfer, Á. Theódórsdóttir, A. Weglewski, P. S. Sorensen, M. Magyari, Initial high-efficacy disease-modifying therapy in multiple sclerosis. Neurology 95, e1041–e1051 (2020).
- A. Rae-Grant, G. S. Day, R. A. Marrie, A. Rabinstein, B. A. C. Cree, G. S. Gronseth, M. Haboubi, J. Halper, J. P. Hosey, D. E. Jones, R. Lisak, D. Pelletier, S. Potrebic, C. Sitcov, R. Sommers, J. Stachowiak, T. S. D. Getchius, S. A. Merillat, T. Pringsheim, Practice guideline recommendations summary: Disease-modifying therapies for adults with multiple sclerosis. Neurology 90, 777–788 (2018).
- H. Wiendl, R. Gold, T. Berger, T. Derfuss, R. Linker, M. Mäurer, O. Aktas, K. Baum, M. Berghoff, S. Bittner, A. Chan, A. Czaplinski, F. Deisenhammer, F. Di Pauli, R. Du Pasquier, C. Enzinger, E. Fertl, A. Gass, K. Gehring, C. Gobbi, N. Goebels, M. Guger, A. Haghikia, H.-P. Hartung, F. Heidenreich, O. Hoffmann, B. Kallmann, C. Kleinschnitz, L. Klotz, V. I. Leussink, F. Leutmezer, V. Limmroth, J. D. Lünemann, A. Lutterotti, S. G. Meuth, U. Meyding-Lamadé, M. Platten, P. Rieckmann, S. Schmidt, H. Tumani, F. Weber, M. S. Weber, U. K. Zettl, T. Ziemssen, F. Zipp, Multiple Sclerosis Therapy Consensus Group (MSTCG): position statement on disease-modifying therapies for multiple sclerosis (white paper). Ther Adv Neurol Disord 14, 17562864211039648 (2021).
- V.-P. Stamatellos, G. Papazisis, Safety and Monitoring of the Treatment with Disease-Modifying Therapies (DMTs) for Multiple Sclerosis (MS). Current Reviews in Clinical and Experimental Pharmacology 18, 39–50 (2023).
- M. Henderson, D. B. Horton, V. Bhise, G. Pal, G. Bushnell, C. V. Dave, Initiation Patterns of Disease-Modifying Therapies for Multiple Sclerosis Among US Adults and Children, 2001 Through 2020. JAMA Neurology 80, 860–867 (2023).
- S. Narayanan, P. O’Meara, J. White, J. Chan, S. Gabriele, E. Hautamaki, Adoption of Oral Disease Modifying Treatments to Manage Patients with Relapsing Remitting Multiple Sclerosis from 2011-2013 in the United States. Value in Health 17, A405 (2014).
- R. J. Desai, M. Mahesri, J. J. Gagne, E. Hurley, A. Tong, T. Chitnis, S. Minden, C. M. Spettell, O. S. Matlin, W. H. Shrank, N. K. Choudhry, Utilization Patterns of Oral Disease-Modifying Drugs in Commercially Insured Patients with Multiple Sclerosis. JMCP 25, 113–121 (2019).
- J. R. Earla, G. J. Hutton, J. D. Thornton, R. R. Aparasu, Factors associated with prescribing oral disease modifying agents in multiple sclerosis: a real-world analysis of electronic medical records. Multiple Sclerosis and Related Disorders 45, 102334 (2020).
- W. J. Culpepper, R. A. Marrie, A. Langer-Gould, M. T. Wallin, J. D. Campbell, L. M. Nelson, W. E. Kaye, L. Wagner, H. Tremlett, L. H. Chen, S. Leung, C. Evans, S. Yao, N. G. LaRocca, on behalf of the United States Multiple Sclerosis Prevalence Workgroup (MSPWG), on behalf of the United States Multiple Sclerosis Prevalence Workgroup (MSPWG), A. Lo, R. McBurney, O. Muravov, B. Talente, L. Ritter, Validation of an algorithm for identifying MS cases in administrative health claims datasets. Neurology 92 (2019).
- I. A. Samjoo, C. Drudge, S. Walsh, S. Tiwari, R. Brennan, I. Boer, D. A. Häring, L. Klotz, N. Adlard, J. Banhazi, Comparative efficacy of therapies for relapsing multiple sclerosis: a systematic review and network meta-analysis. J. Comp. Eff. Res. 12, e230016 (2023).
- X. Montalban, S. L. Hauser, L. Kappos, D. L. Arnold, A. Bar-Or, G. Comi, J. de Seze, G. Giovannoni, H.-P. Hartung, B. Hemmer, F. Lublin, K. W. Rammohan, K. Selmaj, A. Traboulsee, A. Sauter, D. Masterman, P. Fontoura, S. Belachew, H. Garren, N. Mairon, P. Chin, J. S. Wolinsky, Ocrelizumab versus Placebo in Primary Progressive Multiple Sclerosis. New England Journal of Medicine 376, 209–220 (2017).
- S. L. Hauser, A. Bar-Or, J. A. Cohen, G. Comi, J. Correale, P. K. Coyle, A. H. Cross, J. de Seze, D. Leppert, X. Montalban, K. Selmaj, H. Wiendl, C. Kerloeguen, R. Willi, B. Li, A. Kakarieka, D. Tomic, A. Goodyear, R. Pingili, D. A. Häring, K. Ramanathan, M. Merschhemke, L. Kappos, Ofatumumab versus Teriflunomide in Multiple Sclerosis. New England Journal of Medicine 383, 546–557 (2020).
- C. Kang, H. A. Blair, Ofatumumab: A Review in Relapsing Forms of Multiple Sclerosis. Drugs 82, 55–62 (2022).
- R. Bove, T. Chitnis, Sexual disparities in the incidence and course of MS. Clinical Immunology 149, 201–210 (2013).
- L. C. Golden, R. Voskuhl, The importance of studying sex differences in disease: The example of multiple sclerosis. J of Neuroscience Research 95, 633–643 (2017).
- A. Stahmann, E. Craig, D. Ellenberger, F. Fneish, N. Frahm, R. A. Marrie, R. Middleton, R. Nicholas, J. Rodgers, C. Warnke, A. Salter, Disease-modifying therapy initiation patterns in multiple sclerosis in three large MS populations. Ther Adv Neurol Disord 17, 17562864241233044 (2024).
- D. Jakimovski, S. Eckert, R. Zivadinov, B. Weinstock-Guttman, Considering patient age when treating multiple sclerosis across the adult lifespan. Expert Review of Neurotherapeutics 21, 353–364 (2021).
- F. Schweitzer, S. Laurent, G. R. Fink, M. H. Barnett, S. Reddel, H.-P. Hartung, C. Warnke, Age and the risks of high-efficacy disease modifying drugs in multiple sclerosis. Current opinion in neurology 32, 305–312 (2019).
- A. J. Thompson, B. L. Banwell, F. Barkhof, W. M. Carroll, T. Coetzee, G. Comi, J. Correale, F. Fazekas, M. Filippi, M. S. Freedman, Diagnosis of multiple sclerosis: 2017 revisions of the McDonald criteria. The Lancet Neurology 17, 162–173 (2018).
- G. Bsteh, M. L. Aicher, J. F. Walde, N. Krajnc, L. Haider, G. Traxler, C. Gradl, A. Salmen, K. Riedl, P. Poskaite, P. Leyendecker, P. Altmann, M. Auer, K. Berek, F. Di Pauli, B. Kornek, F. Leutmezer, P. S. Rommer, G. Zulehner, T. Zrzavy, F. Deisenhammer, A. Chan, T. Berger, R. Hoepner, H. Hammer, H. Hegen, Association of Disease-Modifying Treatment With Outcome in Patients With Relapsing Multiple Sclerosis and Isolated MRI Activity. Neurology 103, e209752 (2024).
- D. Ontaneda, E. Tallantyre, T. Kalincik, S. M. Planchon, N. Evangelou, Early highly effective versus escalation treatment approaches in relapsing multiple sclerosis. The Lancet Neurology 18, 973–980 (2019).

