Evaluate clinical- and cost- effectiveness to differentiate products using complete EHR data linked with notes and images
Solutions for HEOR
Truveta enables life sciences companies to:
Assess real-world clinical- and cost-effectiveness of therapies to inform market access decisions
Analyze real-world treatment pathways and healthcare resource utilization patterns to inform access and engagement programs
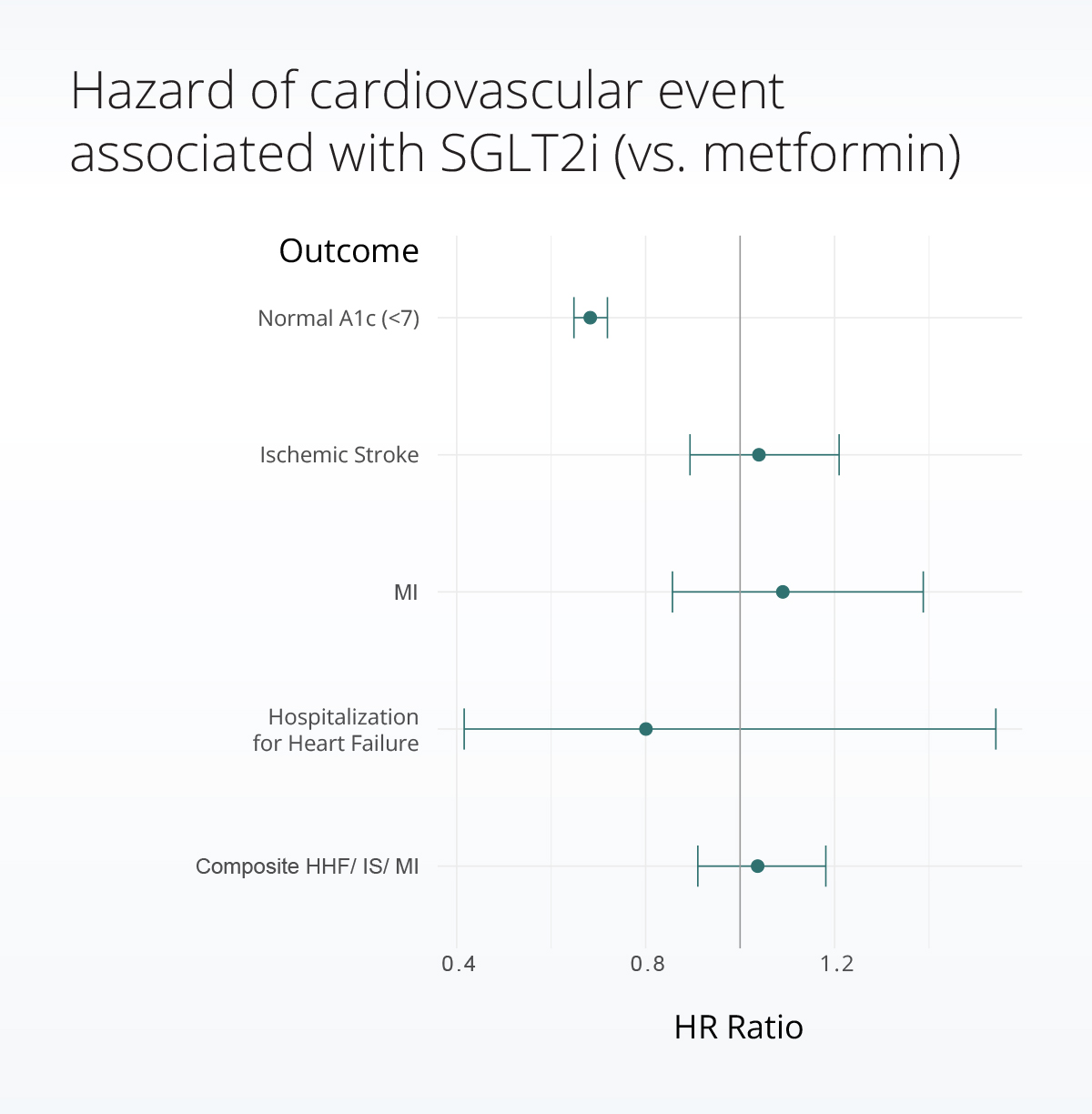
Comparing real-world treatment outcomes
Truveta Data is the most complete, timely, and clean regulatory-grade EHR Data
patients and growing
clinical notes
unique medical devices
years patient history
SDOH attributes
Explore complete, patient-level data
EHR data is linked with SDOH, mortality, and claims data for a complete view into the patient journey.
Stringent data quality standards are met across all data elements, including:














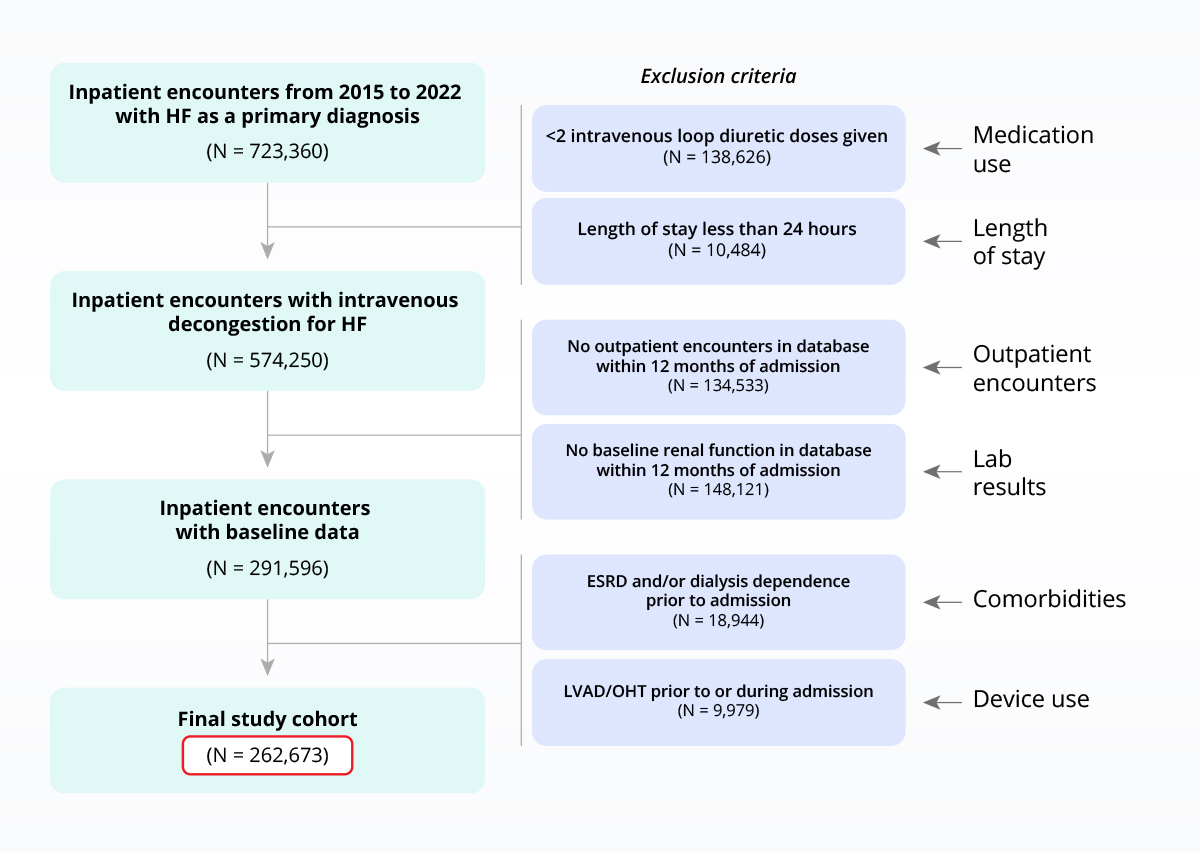
The clinical depth of Truveta Data enables the use of highly-specific inclusion/exclusion criteria.
Every Truveta study can be a health equity study
Truveta Data is linked with SDOH data from LexisNexis Risk Solutions including attributes on education, income, housing stability, social support, and more.
Case study
Comparing the safety of novel pulmonary embolism devices
Real-world data is essential for filling knowledge gaps on drugs and devices. To assess the real-world safety of its EKOS device versus a competitor device, Boston Scientific enlisted independent researchers to compare major bleeding event risks using Truveta Data.
Case study
Comparing the safety of novel pulmonary embolism devices
Real-world data is essential for filling knowledge gaps on drugs and devices. To assess the real-world safety of its EKOS device versus a competitor device, Boston Scientific enlisted independent researchers to compare major bleeding event risks using Truveta Data.