On February 17, 2022, Abbott voluntarily recalled powdered formula made in a Michigan facility due to complaints of Cronobacter sakazakii and Salmonella Newport contamination [1]. Datasembly reported that baby formula was out-of-stock at 31% of stores in April 2022 compared to 23% in January 2022 and between 2-8% during the first 7 months of 2021 [2].
During this time, news outlets showed photos of empty shelves and reported that the end was not in sight for the shortage [3, 4]. As parents were scrambling to find formula for their children, doctors issued specific warnings for what not to do, including watering down infant formula, making “homemade” formula, and buying out-of-country unregulated formula [5]. The Biden Administration took prioritized steps to increase the supply of formula and has reported data showing an increase in cumulative sales of infant formula compared to 2021 [6].
With the recent additional recall of certain Abbott ready-to-feed infant formulas (on 10/14/22) [7, 8], we were interested in exploring the effects of the formula shortage earlier this year, specifically if there was an increase in the rate of newborns and infants with hospital encounters for conditions which may be related to the formula shortage earlier in 2022.
In our data, we saw values higher than expected for newborns experiencing dehydration around the time the formula shortage began. This has prompted additional questions for subgroup analyses. We did not see sustained values outside the expected range in other diagnosis codes throughout the formula shortage.
Methods
We looked at a subset of the Truveta data for newborns (28 days or less) and infants (up to 1 year of age). From January 2021 to August 2022, we included nearly 550,000 newborns and over 850,000 infants. For each day we calculated the rate of diagnosis codes per newborns or infants who met our inclusion criteria (i.e., they were born in the last 28 or 365 days, respectively).
For the newborn population, we included the following ICD10 codes: Dehydration of newborn (P74.1), Hyponatremia of newborn (P74.22), and Feeding problems of newborn (P92*). Hyponatremia — abnormally low levels of sodium in the blood — can occur if an infant is given diluted formula.
For the population of infants under 1 year of age, we included ICD10 codes related to Volume depletion (E86*), Failure to thrive (child) (R62.51), Symptoms and signs concerning food and fluid intake (R63), and Allergy to milk products (Z91.011).
We used temporal smoothing methods to detect anomalies (periods higher than expected) in week-level outcomes for each diagnosis code. Specifically, we selected the lags for each outcome using the standard method of partial autocorrelation. Observed values that fell outside the predicted confidence interval were considered anomalous, meaning they were significantly different from what we expected based on previous information.
Results
Newborns
Overall, when we sum all newborn-related codes together, we see a noisy distribution (i.e., fluctuations over time); however, we did find potentially anomalous values during the same week as the initial formula recall (week of February 13, 2022). In the graph below, the shaded regions demonstrate the 95% confidence interval of our estimate. When values fall outside this region (denoted by the green dots), they are considered potentially anomalous.
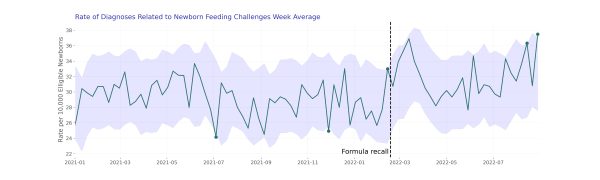
When we dug into the individual diagnosis codes, we saw unexpectedly high dehydration rates contributing to this. We also saw high anomalies of dehydration rates during the weeks of March 6, 2022 and March 13, 2022. Rates during these two weeks were 2.1x higher than rates during the same time-period of 2021. Rates during these two weeks were 2.1x higher than rates during the same time-period of 2021, however it is important to note the population who experienced dehydration is smaller than some of the other diagnosis codes we investigated. Following these increases, we see dehydration rates return to expected values in subsequent weeks.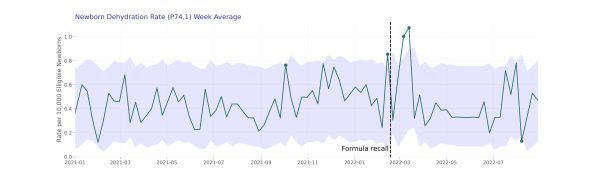
We did not see values outside our expectations for the other two diagnosis codes we investigated (feeding problems of newborns and hyponatremia).
Infants
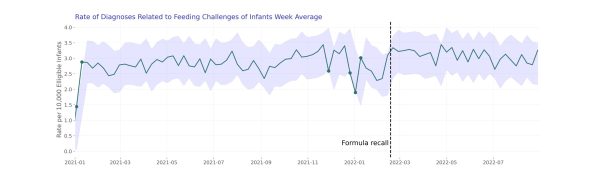
Looking at the sum of rates for infants under 1 year of age, we did not see potentially anomalous values after February 2022. When we looked at each individual rate, we also did not find weekly values that did not match our expectations.
Discussion
Our analysis showed high anomalous values for dehydration rates for newborns during the week of the formula recall and two weeks after. Digging into other diagnostic codes, which may indicate challenges with food access for newborns and infants, we did not see significant high values or sustained high values while the Abbott plant was shut down.
The two-week high anomalous dehydration rates two weeks after the formula shortage started may indicate that immediately after the facility shut down, parents had formula on-hand; however, they ran out within the two-week period. The fact that these trends are not sustained may indicate that parents were able to find solutions to keep their children fed.
Although it is heartening that we do not see sustained increased encounters related to the shortage, this has also prompted more questions for our team, specifically looking at subgroups of newborns and infants affected by the formula shortage:
- The data here are a combination of multiple states; however, a Datasembly analysis indicated that out-of-stock percentages varied by states [2]. Further analysis could explore these differences at the state level.
- There may be disparities in who was affected by the shortage based on social determinants of health factors, such as access to transportation to travel to multiple stores if one was out-of-stock.
- As a combination of the two questions above, we also wonder if there were differences in access based on rural or urban geographies. Were fewer stores out-of-stock of formula for shorter periods of time in urban areas?
- Was there an increase in the rate of prescriptions written for formula during this time-period? Some infants with special nutritional needs require formula prescriptions from their physicians, and it would be interesting to see if prescribing patterns were affected by the formula shortage.
- We are also curious how the healthcare encounter types related to the effects of the formula storage vary by location (i.e., NICU admissions versus emergency department visits). We expect infants admitted to the hospital, including to the NICU, may have other health conditions or more severe conditions that contribute to their overall health and their need for hospital encounters and the impact on formula needs.
- Finally, we expected to see a sustained increase over the months in which formula was challenging to find, rather than only an increase over a few weeks. More research is needed to understand why this effect was not sustained.
With the data presented in this report, we are unable to measure if there was an increase in families requesting or receiving additional support from their doctors or assistant programs, such as Women, Infants, and Children (WIC).
With the timeliness of Truveta’s data, we will continue to monitor the effects of the second formula recall [7] and share any additional impacts on newborn or infant hospital admissions.
These are preliminary research findings and not peer reviewed. Data are constantly changing and updating. These findings are consistent with data pulled October 20, 2022.
Citations
[1] Center for Food Safety and Applied Nutrition. “Abbott Voluntarily Recalls Powder Formulas Manufactured at One Plant.” U.S. Food and Drug Administration, FDA, 17 Feb. 2022, https://www.fda.gov/safety/recalls-market-withdrawals-safety-alerts/abbott-voluntarily-recalls-powder-formulas-manufactured-one-plant.
[2] Datasembly. “Datasembly’s Data Reveals 31% out-of-Stock Rate in April 2022 for Baby Formula; up 11% Compared to November 2021.” Datasembly, 29 June 2022, https://datasembly.com/news/datasemblys-data-reveals-31-out-of-stock-rate-in-april-2022-for-baby-formula-up-11-compared-to-november-2021/.
[3] Wile, Rob. “40 percent of American’s baby formula supplies are out of stock.” NBC News, 9 May 2022, https://www.cnbc.com/2022/05/09/40-percent-of-americas-baby-formula-supplies-are-out-of-stock.html.
[4] Gasparro, Annie and Jaewon Kang. “Baby Formula Shortage Could Leave Parents Scrambling for Months; Formula makers and retailers say it will take months to address long-running shortages; Congress schedules hearing.” WSJ, 12 May 2022, https://www.wsj.com/articles/baby-formula-shortage-could-last-months-11652371827.
[5] Memorial Health. “Infant Formula Shortage: What Not to Do.” Memorial Health, 2 June, 2022, https://blog.memorial.health/infant-formula-shortage-what-not-to-do/.
[6] The White House. “Addressing the Infant Formula Shortage.” The White House, 17 Oct. 2022, https://www.whitehouse.gov/FORMULA/.
[7] Center for Food Safety and Applied Nutrition. “Abbott Voluntarily Recalls Certain Lots of 2 Fl. Oz./59 mL Bottles of Ready-to-Feed Liquid Products; Recall Is Not Expected to Impact U.S. Infant Formula Supply.” U.S. Food and Drug Administration, FDA, 14 Oct. 2022, https://www.fda.gov/safety/recalls-market-withdrawals-safety-alerts/abbott-voluntarily-recalls-certain-lots-2-fl-oz59-ml-bottles-ready-feed-liquid-products-recall-not.
[8] Abbott. “Abbott Voluntarily Recalls Certain Lots of 2 Fl. Oz./59 mL Bottles of Ready-to-Feed Liquid Products; Recall Is Not Expected to Impact U.S. Infant Formula Supply.”14 Oct. 2022, https://www.abbott.com/corpnewsroom/nutrition-health-and-wellness/voluntary-recall-of-certain-2-fl-oz-liquid-products.html.
