The National Institute of Health estimates that more than six million Americans over the age of 65 suffer from Alzheimer’s disease, with many more under the age of 65 also having the disease (National Institute on Aging, 2021). It is currently ranked as the seventh leading cause of death in the United States. While there is no cure for Alzheimer’s disease, there are some treatments available to help manage the disease symptoms (National Institute on Aging, 2021). Most recently, the initial findings from a clinical trial studying the effectiveness of lecanemab (sold as Leqembi) to slow Alzheimer’s Disease progression were reported in September 2022 (Eisai, 2022; van Dyck et al., 2023). Since then, the Federal Food and Drug Administration granted accelerated approval of the drug for people with mild cognitive impairment or early Alzheimer’s disease in early January 2023 (FDA, 2023; Lovelace Jr., 2023).
Results from the clinical trial suggest that some people may experience more adverse side effects from the new therapy. Specifically, among people treated with lecanemab, those who have APOE ε4 experienced a higher incidence of brain swelling and/or small hemorrhages, collectively known as amyloid-related imaging abnormalities (ARIA), than non-carriers. APOE is the gene that encodes the protein apolipoprotein E, which plays a role in lipid transport and metabolism. The APOE ε3 isoform is the most common and is considered the “typical” form, while ε2 and ε4 are considered variants. EISAI and Biogen, the companies that developed lecanemab, suggest clinicians consider testing for APOE ε4 status to inform treatment decisions (Eisai Global, 2023).
To date, access to lecanemab has been limited due to a decision by CMS (Medicare) to offer limited coverage of the drug until it has full FDA approval, which is expected in July 2023 (Centers for Medicare & Medicaid Services, 2023). Still, changes in tests for APOE ε4 may be suggestive of intention to seek treatment with lecanemab.
We worked exclusively with reporter Julie Steenhuysen at Reuters to explore the trends in APOE genetic testing.
Methods
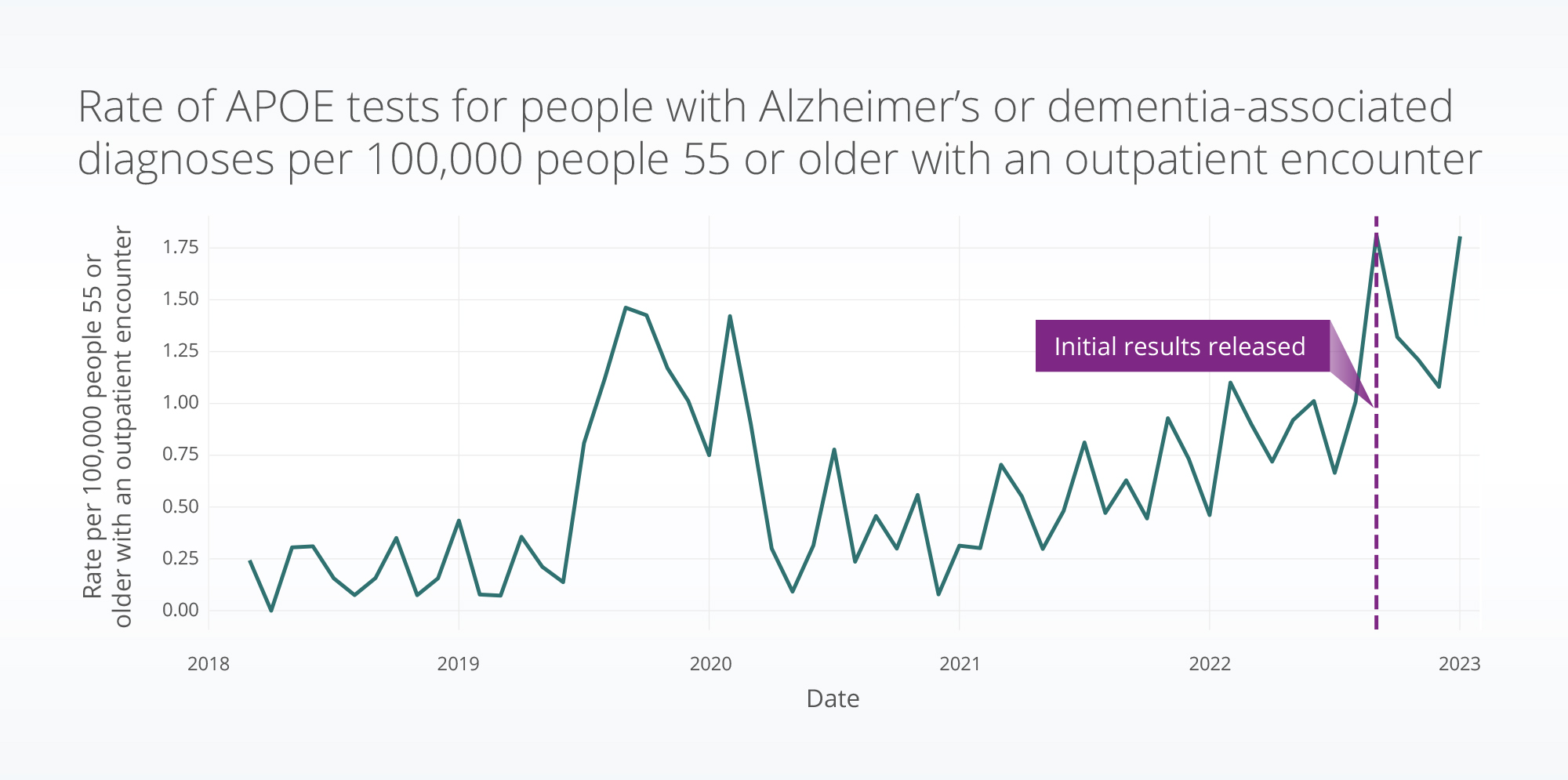
Using a subset of Truveta data, we identified patients who had an APOE test between January 1, 2018 – January 31, 2023. APOE tests were defined using four active LOINC codes representing APOE genotype testing.
To account for the fact that APOE tests are conducted for other disease areas besides Alzheimer’s or dementia, such as hyperlipidemia, we found the diagnostic codes associated with the APOE lab tests and classified them as Alzheimer’s/dementia-related or related to other diseases. Some patients had both indications for Alzheimer’s/dementia and other diseases (such as hyperlipidemia). We created a subset of the overall population that included patients with an Alzheimer’s/dementia-related diagnosis; patients were counted regardless of whether they had other indications.
We found the rate of APOE tests with an associated Alzheimer’s/dementia-related diagnosis per 100,000 people aged 55 or older with an outpatient encounter each month.
Results
Based on our analysis, the monthly average of the rate of APOE testing from September 2022 – January 2023 increased 125% compared with one year prior (September 2021 – January 2022). The September 2022 – January 2023 rate averaged 1.4 per 100,000 outpatient encounters for people aged 55 or older, compared with the September 2021 – January 2022 rate of 0.6 per 100,000 encounters. This is a 2.3x increase for the same period year over year.
Discussion
The increase in APOE genetic testing in September 2022 – January 2023 compared to one year prior may suggest that there is an increase in this testing due to the desire to pursue treatment with lecanemab.
There was also a substantial increase in APOE testing for this population during mid-2019. In July 2019, a land-mark study was published in Nature reviewing the literature and discussing how the APOE gene could be targeted to treat Alzheimer’s disease (Yamazaki et al., 2019). This may have contributed to the increase in testing, especially since we saw an increase, until roughly March 2020, when the pandemic began.
We will continue to follow these trends, as well as trends related to lecanemab access and use, especially following the full approval expected in July 2023.
Read the full story in Reuters about this topic: ‘It totally backfired’: The pitfalls of Alzheimer’s genetic testing.
These are preliminary research findings and not peer reviewed. Data are constantly changing and updating. These findings are consistent with data pulled February 23, 2023.
References
Centers for Medicare & Medicaid Services. (2023, February 22). CMS STATEMENT: Response to Alzheimer’s Association’s Request to Reconsider the Final National Coverage Determination. https://www.cms.gov/newsroom/press-releases/cms-statement-response-alzheimers-associations-request-reconsider-final-national-coverage
Eisai. (2022). LECANEMAB CONFIRMATORY PHASE 3 CLARITY AD STUDY MET PRIMARY ENDPOINT, SHOWING HIGHLY STATISTICALLY SIGNIFICANT REDUCTION OF CLINICAL DECLINE IN LARGE GLOBAL CLINICAL STUDY OF 1,795 PARTICIPANTS WITH EARLY ALZHEIMER’S DISEASE. https://media-us.eisai.com/2022-09-27-LECANEMAB-CONFIRMATORY-PHASE-3-CLARITY-AD-STUDY-MET-PRIMARY-ENDPOINT,-SHOWING-HIGHLY-STATISTICALLY-SIGNIFICANT-REDUCTION-OF-CLINICAL-DECLINE-IN-LARGE-GLOBAL-CLINICAL-STUDY-OF-1,795-PARTICIPANTS-WITH-EARLY-ALZHEIMERS-DISEASE
Eisai Global. (2023, March 6). FDA Accepts Eisai’s Filing of a Supplemental Biologics License Application and Grants Priority Review for Traditional Approval of LEQEMBITM (lecanemab-irmb) for the Treatment of Alzheimer’s Disease. https://www.eisai.com/news/2023/news202316.html
FDA. (2023, January 6). FDA Grants Accelerated Approval for Alzheimer’s Disease Treatment. U.S. Food & Drug Administration. https://www.fda.gov/news-events/press-announcements/fda-grants-accelerated-approval-alzheimers-disease-treatment
Lovelace Jr., B. (2023, January 6). FDA approves new Alzheimer’s drug that appears to slow progression of disease. https://www.nbcnews.com/health/health-news/fda-approves-alzheimers-drug-appears-slow-disease-rcna62928
National Institute on Aging. (2021, July 8). Basics Of Alzheimer’s Disease And Dementia What Is Alzheimer’s Disease? https://www.nia.nih.gov/health/what-alzheimers-disease
van Dyck, C. H., Swanson, C. J., Aisen, P., Bateman, R. J., Chen, C., Gee, M., Kanekiyo, M., Li, D., Reyderman, L., Cohen, S., Froelich, L., Katayama, S., Sabbagh, M., Vellas, B., Watson, D., Dhadda, S., Irizarry, M., Kramer, L. D., & Iwatsubo, T. (2023). Lecanemab in Early Alzheimer’s Disease. New England Journal of Medicine, 388(1), 9–21. https://doi.org/10.1056/NEJMoa2212948
Yamazaki, Y., Zhao, N., Caulfield, T. R., Liu, C.-C., & Bu, G. (2019). Apolipoprotein E and Alzheimer disease: Pathobiology and targeting strategies. Nature Reviews Neurology, 15(9), 501–518. https://doi.org/10.1038/s41582-019-0228-7
