Authors: Duy Do, PhD ⊕Truveta, Inc, Bellevue, WA, Patricia J. Rodriguez, PhD, MPH ⊕Truveta, Inc, Bellevue, WA, Brianna M. Goodwin Cartwright, MS ⊕Truveta, Inc, Bellevue, WA, Nicholas Stucky, MD, PhD ⊕Truveta, Inc, Bellevue, WA
- Moderate adherence was observed for patients prescribed injectable cabotegravir, both in combination with rilpivirine for HIV treatment (68%) and as a standalone option for pre-exposure prophylaxis (PrEP) (51%).
- A notable proportion of individuals, across both treatment and PrEP cohorts (8% to 13%), did not complete the initial two-month initiation phase of injectable cabotegravir, highlighting a potential challenge in the early stages of treatment or prevention.
- Adherence patterns following the initiation phase varied across different demographic and clinical characteristics, including age, sex, race, education level, and comorbidity status, suggesting the need for tailored approaches to support adherence in specific patient populations.
This blog is an extension of our poster presented at ISPOR 2025, titled Characteristics and Adherence of Patients Initiating Injectable Cabotegravir for HIV Treatment or Prevention.
Injectable cabotegravir, both as a combination with rilpivirine for HIV treatment and as a standalone for pre-exposure prophylaxis (PrEP), represents a significant advancement in HIV care and prevention. Traditional oral HIV medications require daily adherence, which can be challenging for some individuals, leading to suboptimal viral suppression and reduced PrEP effectiveness (1). Long-acting injectable formulations, like cabotegravir, offer the potential to overcome these adherence barriers by providing sustained drug levels with less frequent dosing. Clinical trials have demonstrated the efficacy and safety of injectable cabotegravir (2–4), but understanding its real-world uptake and adherence patterns is crucial for optimizing its impact on the HIV epidemic. Factors such as patient demographics, access to care, and individual preferences may influence adherence to injectable regimens (5).
This analysis utilizes real-world data to describe the characteristics of individuals initiating injectable cabotegravir for treatment and prevention and to evaluate their adherence to the prescribed regimens. The findings from this study will provide valuable insights into the implementation of long-acting injectable cabotegravir in routine clinical practice, helping to identify potential challenges and inform strategies to maximize its benefits for individuals at risk for or living with HIV.
You can view the full code and data definitions directly in Truveta Studio.
Methods
This study utilized a subset of Truveta Data. Two cohorts were defined: 1) An HIV treatment cohort and 2) HIV prevention cohort. Patients in both cohorts were required to be over 18 years of age. The HIV treatment cohort was defined as patients who initiated injectable cabotegravir/rilpivirine between January 2021 and June 2024 and had a prior HIV diagnosis. Patients in the HIV prevention cohort initiated injectable cabotegravir for HIV prevention (PrEP) between December 2021 and June 2024 and had no prior diagnosis for HIV. To ensure a consistent baseline of care, all participants, in both cohorts, had at least one outpatient visit within a year before the first injection and were followed up for six months after the first injection to assess adherence outcomes.
Adherence outcomes were measured based on the following criteria:
- Dropout was defined as not completing two initiation injections within the first 2 months, reflecting those who did not successfully begin the long-acting regimen.
- Non-adherence was defined as missing scheduled injections post-initiation, indicating interruptions in ongoing treatment or prevention.
- Adherence was defined as following the injection schedule, including planned oral bridging therapy, with the treatment cohort following a schedule of every 2 months or once monthly (depending on the regimen), and the prevention cohort following an every-2-month schedule.
Descriptive statistics were used to characterize the cohorts and report adherence outcomes, providing an overview of patient demographics and adherence patterns.
Results
The HIV treatment cohort was comprised of 1,226 adults, while the HIV prevention cohort consisted of 831 adults.
The prevalence of adherence to injectable cabotegravir varied between the HIV treatment and prevention cohorts of U.S. adults aged 18 and older (figure 1).
In the HIV treatment cohort (N=1,226), the majority (68%) of individuals demonstrated adherence to the prescribed injection schedule over the six-month follow-up period. However, a notable proportion experienced non-adherence (24%), and 8% did not complete the initial two-month initiation phase, defined as dropout.
The prevention cohort (PrEP, N=831) showed a lower rate of adherence at 51%, with a larger proportion experiencing non-adherence (36%) and a higher dropout rate (13%) during the initiation phase compared to the treatment cohort.
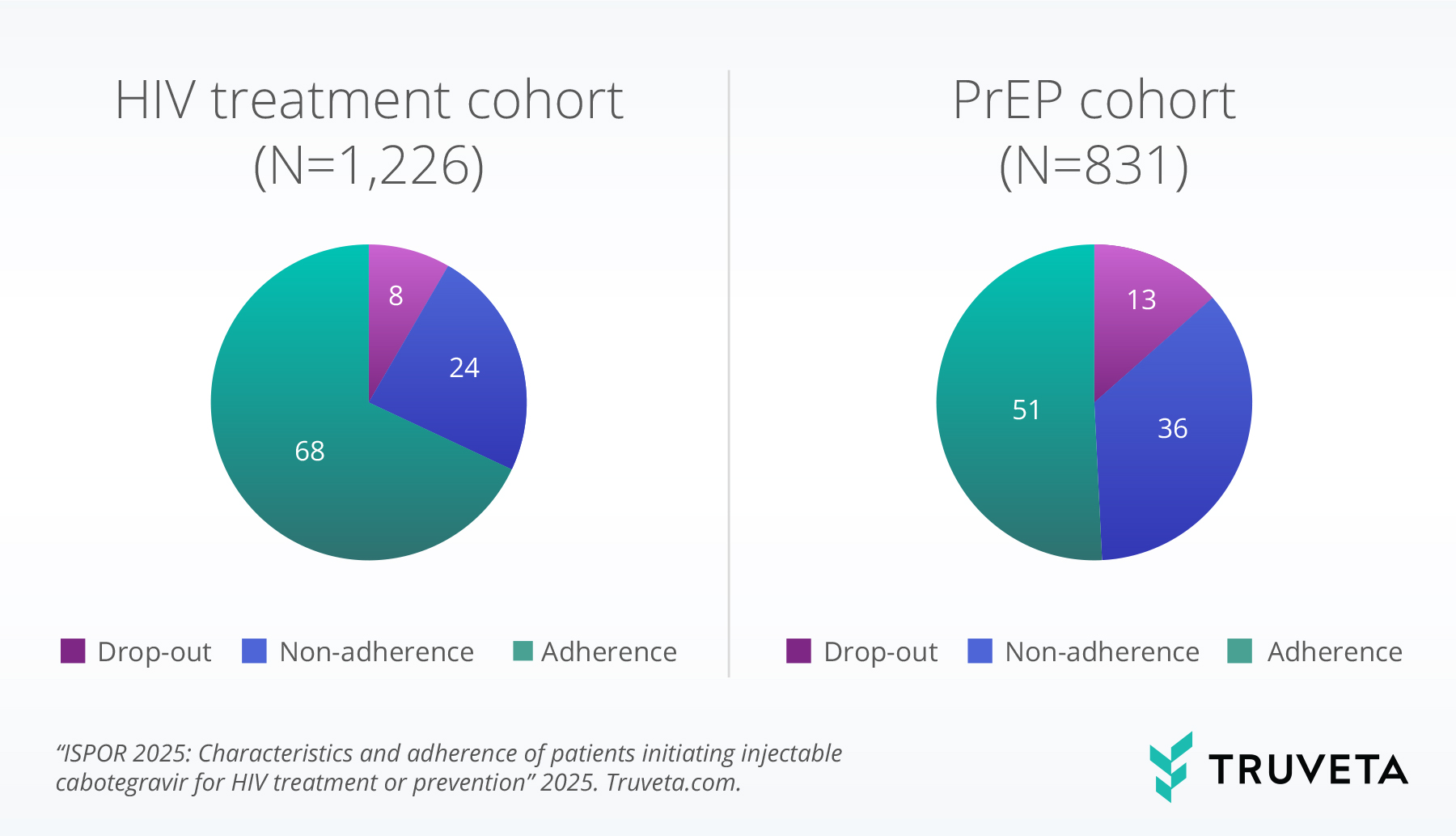
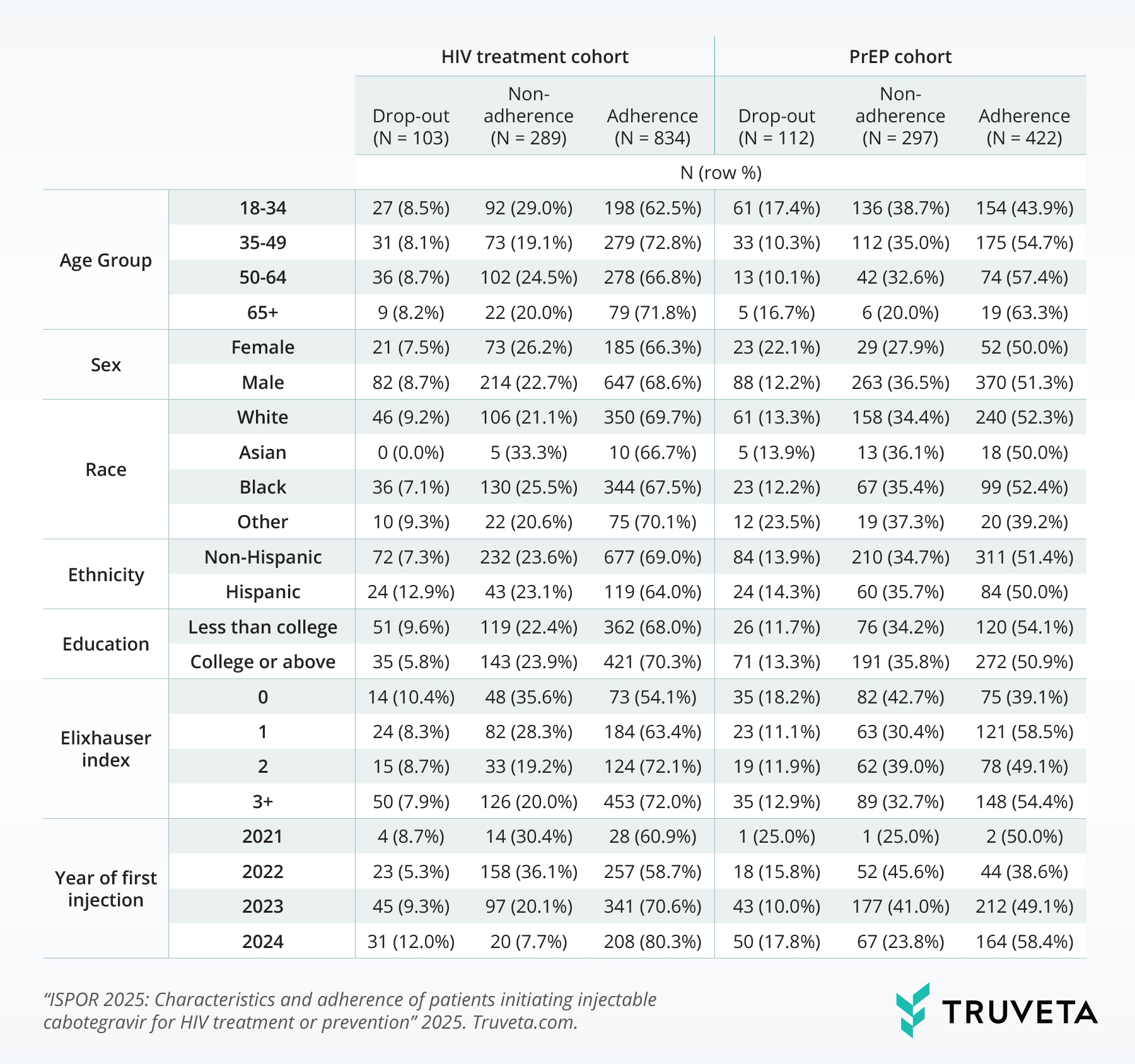
Table 1 presents the prevalence of dropout, non-adherence, and adherence to injectable cabotegravir for HIV treatment and prevention across various demographic and clinical characteristics. In the HIV treatment cohort, adherence appeared to increase with age, reaching 71.8% in the 65+ age group, while dropout was highest in the 50-64 age range (8.7%). Males in the treatment cohort exhibited higher adherence (68.6%) compared to females (66.3%). White individuals showed the highest adherence (69.7%) among racial groups, while Black or African American (henceforth referred to as Black), individuals had the highest non-adherence rate (25.5%). Individuals with a college education or above demonstrated slightly higher adherence (70.3%) compared to those with less than a college education (68.0%). Adherence appeared to increase with a higher Elixhauser comorbidity index, with those having three or more comorbidities showing the highest adherence (72.0%). Furthermore, adherence rates in the treatment cohort increased over the study period, from 60.9% for those initiating in 2021 to 80.3% for those initiating in 2024.
Similar to the treatment cohort, adherence tended to increase with age in the PrEP cohort, reaching 63.3% in the 65+ group. However, the trend was less pronounced. Males in the PrEP cohort also showed higher adherence (51.3%) compared to females (50.0%). White individuals had the highest adherence (52.3%) among racial groups in the PrEP cohort, while Black individuals exhibited the highest non-adherence rate (35.4%). Educational attainment showed a less clear association with adherence in the PrEP cohort. The Elixhauser comorbidity index did not demonstrate a consistent trend with adherence in the PrEP cohort. Similar to the treatment cohort, adherence rates in the PrEP cohort appeared to increase over time, from 38.6% for those initiating in 2022 to 58.4% for those initiating in 2024; the number of individuals initiating in 2021 was small given the late 2021 approval for HIV prevention.
Table 1: Prevalence of drop-out, non-adherence, and adherence for of U.S. adults ages 18 and older initiating cabotegravir for HIV treatment and prevention, by selected characteristics.
Discussion
The findings of this real-world study highlight distinct adherence patterns to long-acting injectable cabotegravir. While a moderate proportion of individuals who successfully completed the initial two-month initiation phase demonstrated adherence to the ongoing regimen for both HIV treatment and PrEP, a notable proportion did not complete the initiation phase. This observation supports the potential of long-acting cabotegravir to address challenges associated with daily oral medication adherence and improve viral suppression and PrEP effectiveness among those who continue with injections. However, the significant dropout rate during initiation highlights a critical period where targeted interventions are necessary to ensure successful uptake of this treatment modality.
Several demographic and clinical characteristics appear to influence adherence patterns. In both the treatment and PrEP cohorts, adherence tended to increase with age, a finding that may reflect differences in healthcare-seeking behavior, life priorities, or support systems across age groups. The higher adherence rates observed among male participants compared to female participants warrant further exploration to understand potential gender-specific barriers to care or treatment preferences. Racial disparities were also evident, with white individuals demonstrating the highest adherence rates and Black individuals exhibiting the highest non-adherence rates, underscoring the persistent need to address systemic inequities in healthcare access and delivery (6).
Interestingly, in the HIV treatment cohort, adherence appeared to improve with an increasing Elixhauser comorbidity index. This counterintuitive finding may suggest that individuals with more complex health conditions receive more intensive clinical management and support, leading to better adherence to their HIV medications. Furthermore, a significant increase in adherence rates was observed over the study period for both cohorts, potentially indicating improvements in patient selection, provider experience, or the availability of support services as long-acting cabotegravir was more widely adopted. These findings collectively emphasize the importance of tailoring support strategies to individual patient needs and addressing potential barriers to optimize the real-world effectiveness of long-acting injectable cabotegravir.
These findings are consistent with data accessed on January 8, 2025.
Citations
- D. T. Dimitrov, B. R. Mâsse, D. Donnell, PrEP Adherence Patterns Strongly Affect Individual HIV Risk and Observed Efficacy in Randomized Clinical Trials. JAIDS Journal of Acquired Immune Deficiency Syndromes 72 (2016).
- M. N. Ramgopal, A. Castagna, C. Cazanave, V. Diaz-Brito, R. Dretler, S. Oka, O. Osiyemi, S. Walmsley, J. Sims, G. Di Perri, Efficacy, safety, and tolerability of switching to long-acting cabotegravir plus rilpivirine versus continuing fixed-dose bictegravir, emtricitabine, and tenofovir alafenamide in virologically suppressed adults with HIV, 12-month results (SOLAR): a randomised, open-label, phase 3b, non-inferiority trial. The Lancet HIV 10, e566–e577 (2023).
- R. J. Landovitz, D. Donnell, M. E. Clement, B. Hanscom, L. Cottle, L. Coelho, R. Cabello, S. Chariyalertsak, E. F. Dunne, I. Frank, Cabotegravir for HIV prevention in cisgender men and transgender women. New England Journal of Medicine 385, 595–608 (2021).
- S. Delany-Moretlwe, J. P. Hughes, P. Bock, S. G. Ouma, P. Hunidzarira, D. Kalonji, N. Kayange, J. Makhema, P. Mandima, C. Mathew, Cabotegravir for the prevention of HIV-1 in women: results from HPTN 084, a phase 3, randomised clinical trial. The Lancet 399, 1779–1789 (2022).
- Truveta Research, Real-world prescribing of long-acting injectable cabotegravir for treatment of HIV in the United States (2023). https://www.truveta.com/blog/research/prescribing-of-cabotegravir-hiv/.
- S. Mahajan, C. Caraballo, Y. Lu, J. Valero-Elizondo, D. Massey, A. R. Annapureddy, B. Roy, C. Riley, K. Murugiah, O. Onuma, Trends in differences in health status and health care access and affordability by race and ethnicity in the United States, 1999-2018. Jama 326, 637–648 (2021).


