- Adequate treatments are a crucial piece in the fight against the spread of HIV, because they can make HIV viral load undetectable in the blood and thus untransmittable. Approved by the US Food and Drug Administration (FDA) in January 2021, one of the newest treatments – cabotegravir/rilpivrine – is the first long-acting injectable HIV treatment.
- Prior to this treatment being available, the only therapies available were daily oral therapies. Little data exists on who is being prescribed cabotegravir in the real-world and if there are barriers to access. We compared a population of people with first-time cabotegravir and bictagravir/emtricitabine/tenofovir alafenamide (most common oral therapy).
- We found people with first-time cabotegravir prescriptions are more likely to be younger, have a longer HIV duration, have a higher baseline CD4, have a higher individual income, and are more likely to have attended college than those first prescribed bictegravir. Bictegravir is still prescribed over 2x more often than cabotegravir. These results suggest, there may be barriers to accessing cabotegravir for a population with HIV.
This blog is an extension of our poster presented at ISPOR Europe 2023 titled Real-World Prescribing of Long-Acting Injectable Cabotegravir for Treatment of HIV in the United States.
For the more than 1.2 million people living with human immunodeficiency virus (HIV) in the United States, the goal of consistently taking antiretroviral therapy (ART) is to achieve an undetectable HIV viral load in the blood (Centers for Disease Control and Prevention, 2023a). This both keeps a person healthier and essentially eliminates the risk of transmission (National Institute on Allergy and Infectious Diseases, 2019). Prior to 2021, the only treatments available were oral daily pills; however, in January 2021, the FDA approved the first extended-release injectable treatment (cabotegravir and rilpivrine (Cabenuva), henceforth referred to as cabotegravir) (US Food & Drug Administration, 2021a).
This new treatment may increase convenience for people who had previously struggled to take a once-daily pill. However, the increased cost of cabotegravir (up to USD$22,2000 for the first year (Sharfstein et al., 2022)) compared to the most common oral treatment, bictagravir/emtricitabine/tenofovir alafenamide (henceforth referred to as bictegravir), remains high and may create challenges in access. Little data exists in understanding the prescribing trends, demographics, and social drivers of health (SDOH) for people who receive a new prescription for cabotegravir compared to bictagravir.
Methods
Using a subset of Truveta Data, we identified patients with HIV who had a first-time prescription for either cabotegravir or bictegravir between January 2021 and June 2023 (if patient had previously received bictegravir, but switched to cabotegravir during the study period, they were eligible for the cabotegravir population). Patients were excluded if they did not receive usual care, defined as at least one encounter within each of the two years prior to initiating the prescription.
We describe patient demographics, comorbidities, SDOH, and most recent CD4 (count per microliter) within the 12 months prior to initiation. CD4 is a count of how many CD4 cells are in the blood and is used as a measure of immune function (Centers for Disease Control and Prevention, 2021). HIV reduces the quantity of CD4 by attacking the cells in the bloodstream, impairing the body’s ability to combat infections. High CD4 counts indicate the body’s ability to fight infections(Centers for Disease Control and Prevention, 2021).
Results
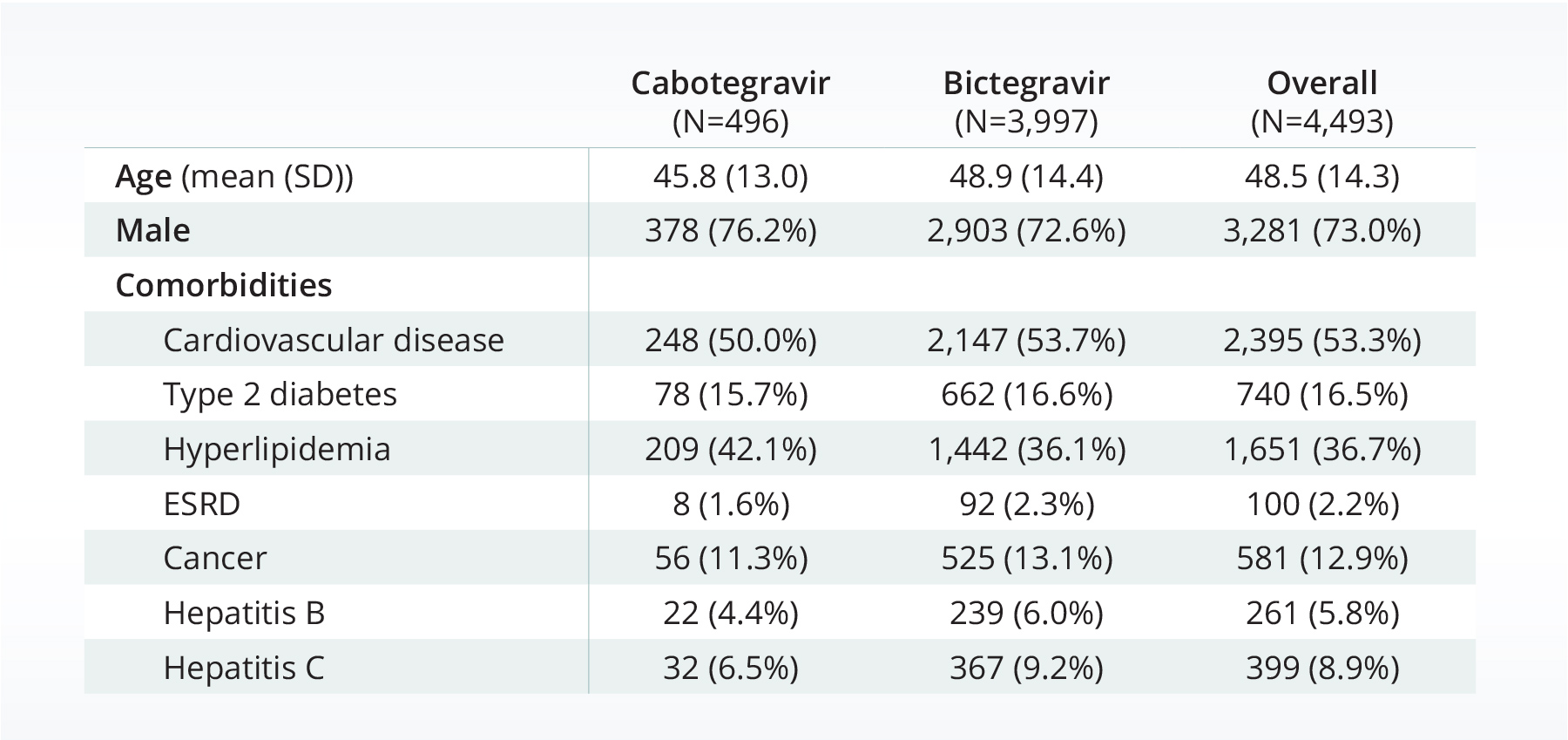
Of the 4,493 patients who met our inclusion criteria, only 11% of the population was prescribed cabotegravir. The population with first-time prescriptions of cabotegravir were younger on average than the population who received bictegravir (45.8 years, compared to 48.9 years). Both populations were primarily male (76.2% for cabotegravir and 72.6% for bictegravir).
HIV and other comorbidities
The population with first-time cabotegravir prescriptions had HIV for an average of one year longer than those with first-time bictegravir prescriptions (4.13 years compared to 3.09 years). The plots below are box plots (or box-and-whisker plots) provide insight into the distribution of data points. The middle line shows the median value, while the box encapsulates the quartiles within the datasets. The outer lines (whiskers) extend to the maximum and minimum data points, which are not considered outliers. If outliers exist, they are shown as dots.
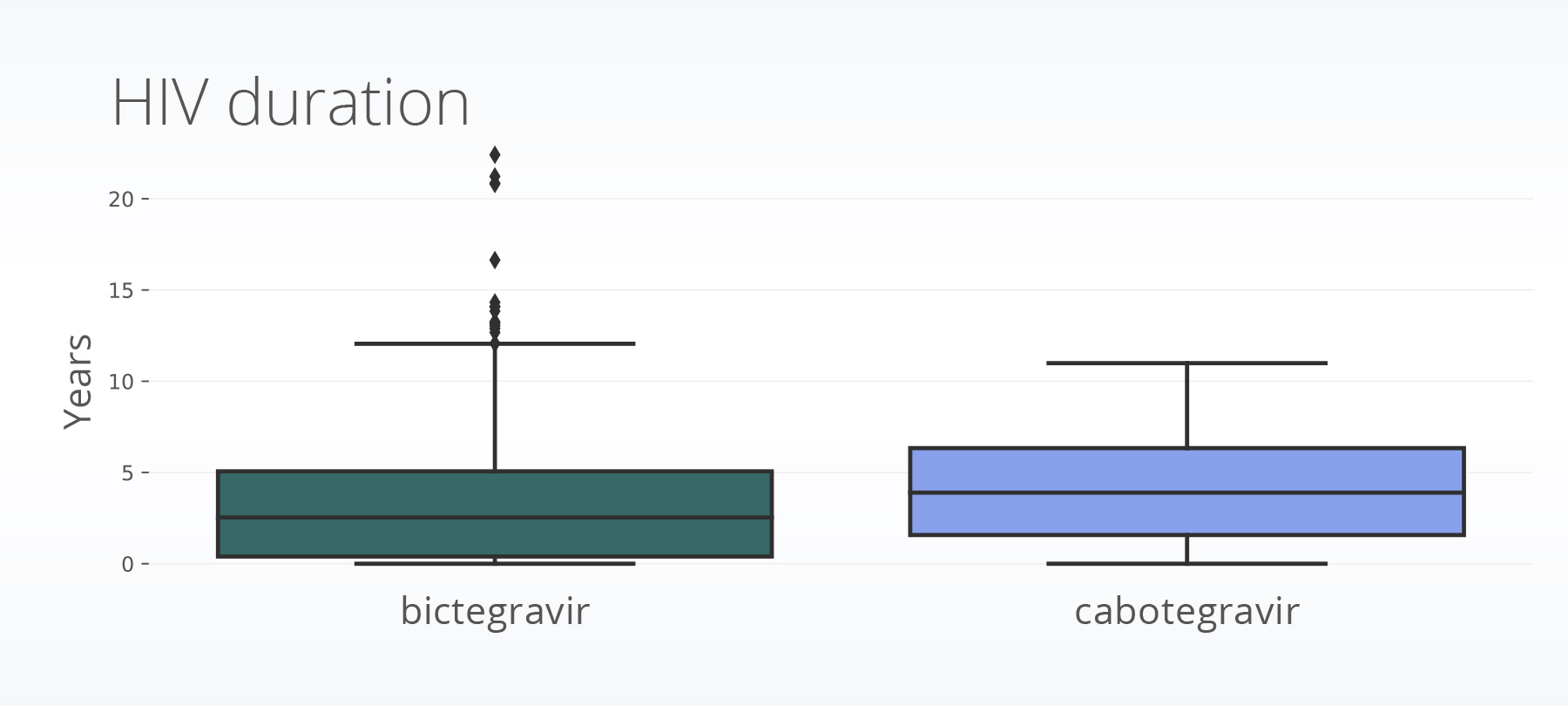
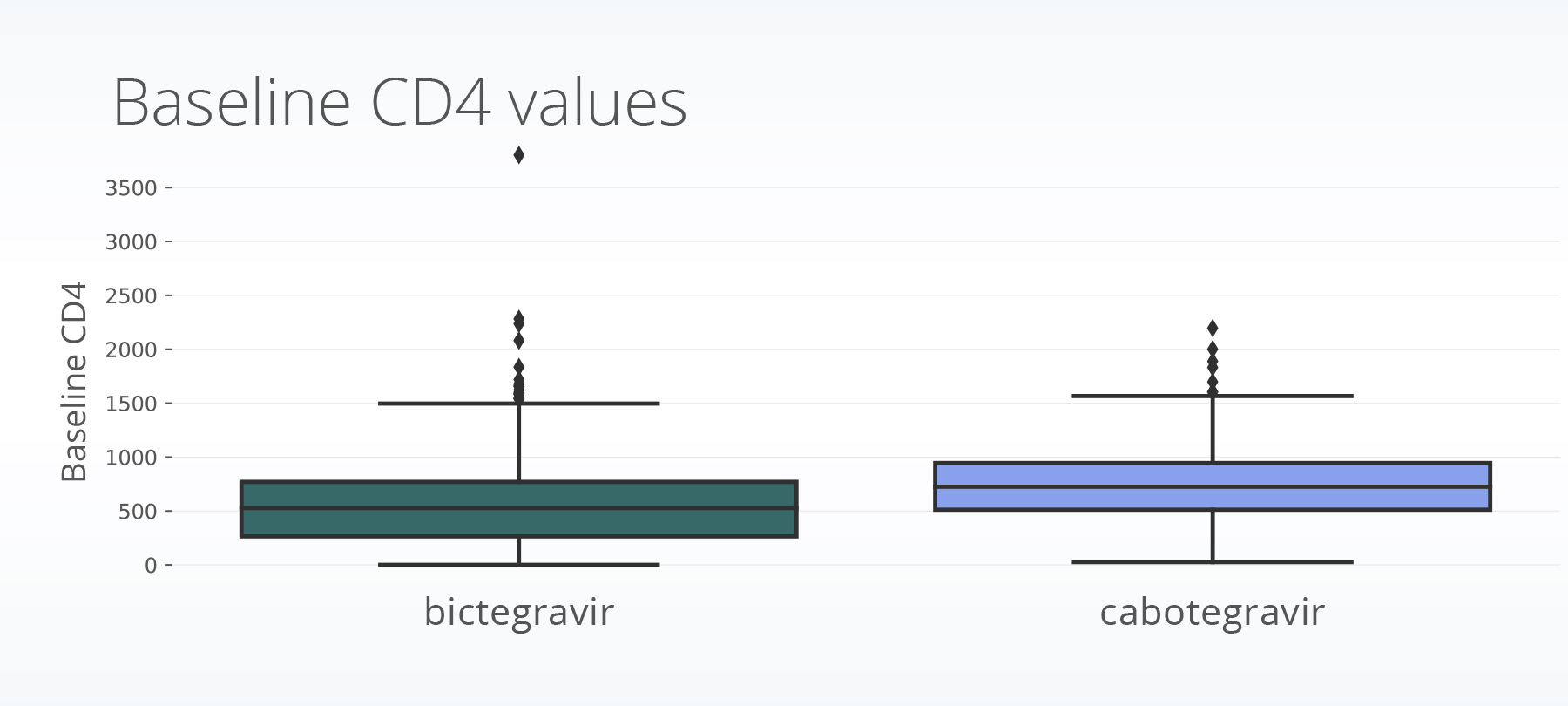
The cabotegravir population had less missing baseline CD4 data (43.5% compared to 67.4%) and higher baseline CD4 values than the population receiving bictegravir (cabotegravir: 755; bictegravir: 550).
The percentage of people with AIDS in each group were similar (cabetgravir: 20.8%; bictegravir 21.8%). Both populations had high rates of cardiovascular disease (50% or over), moderate levels of hyperlipidemia, and moderate levels of type 2 diabetes.
Social drivers of health (SDOH)
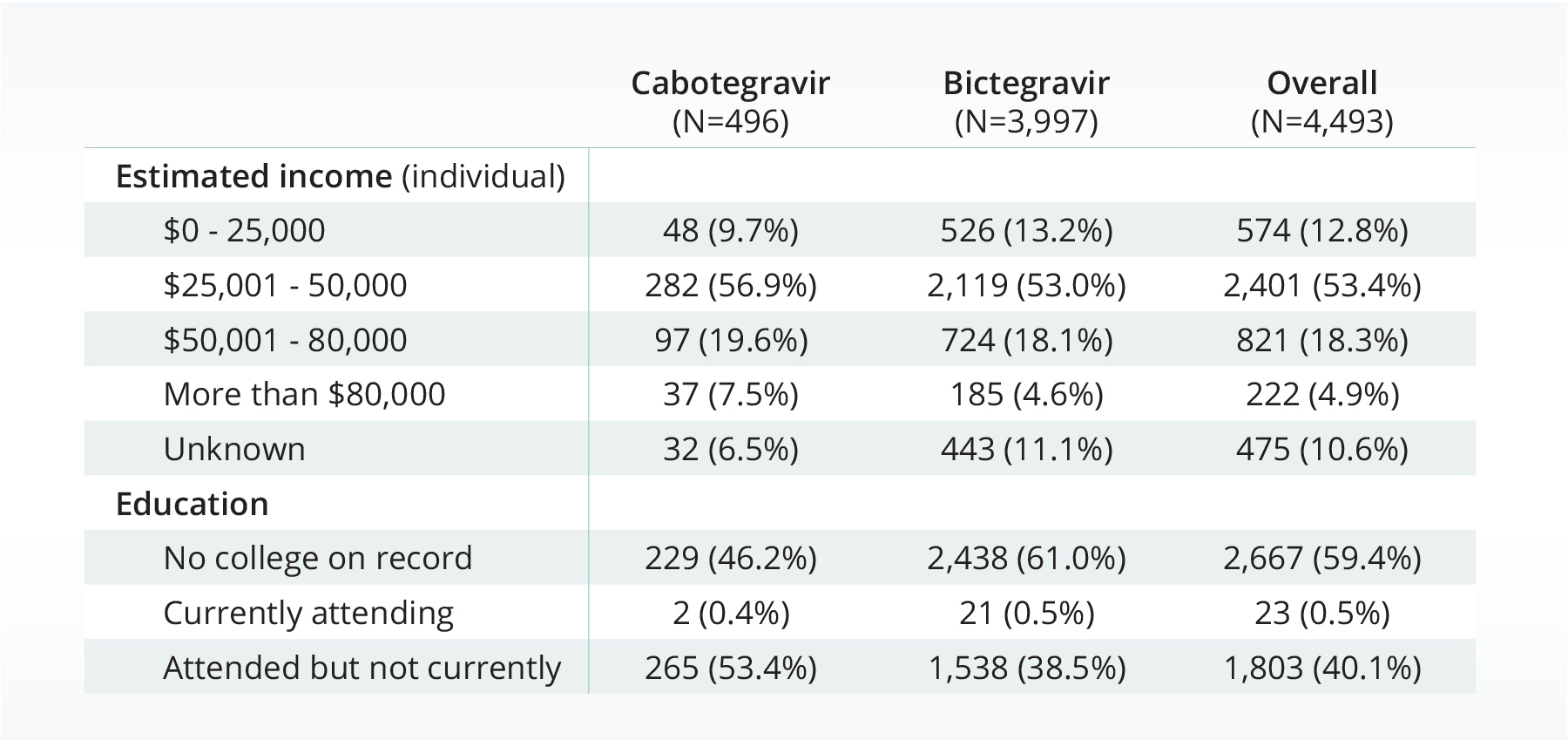
A higher proportion of the cabotegravir population made more than USD$80,000 per year and was either currently or previously attending college compared to the bictegravir population.
Prescription Trends
The ratio of cabotegravir to bictegravir prescriptions remained low through 2021 (less than .1). In 2022 – 2023, the ratio has increased; however, prescriptions still remain more than 2x less for the injectable cabotegravir compared to the oral bictegravir treatments.
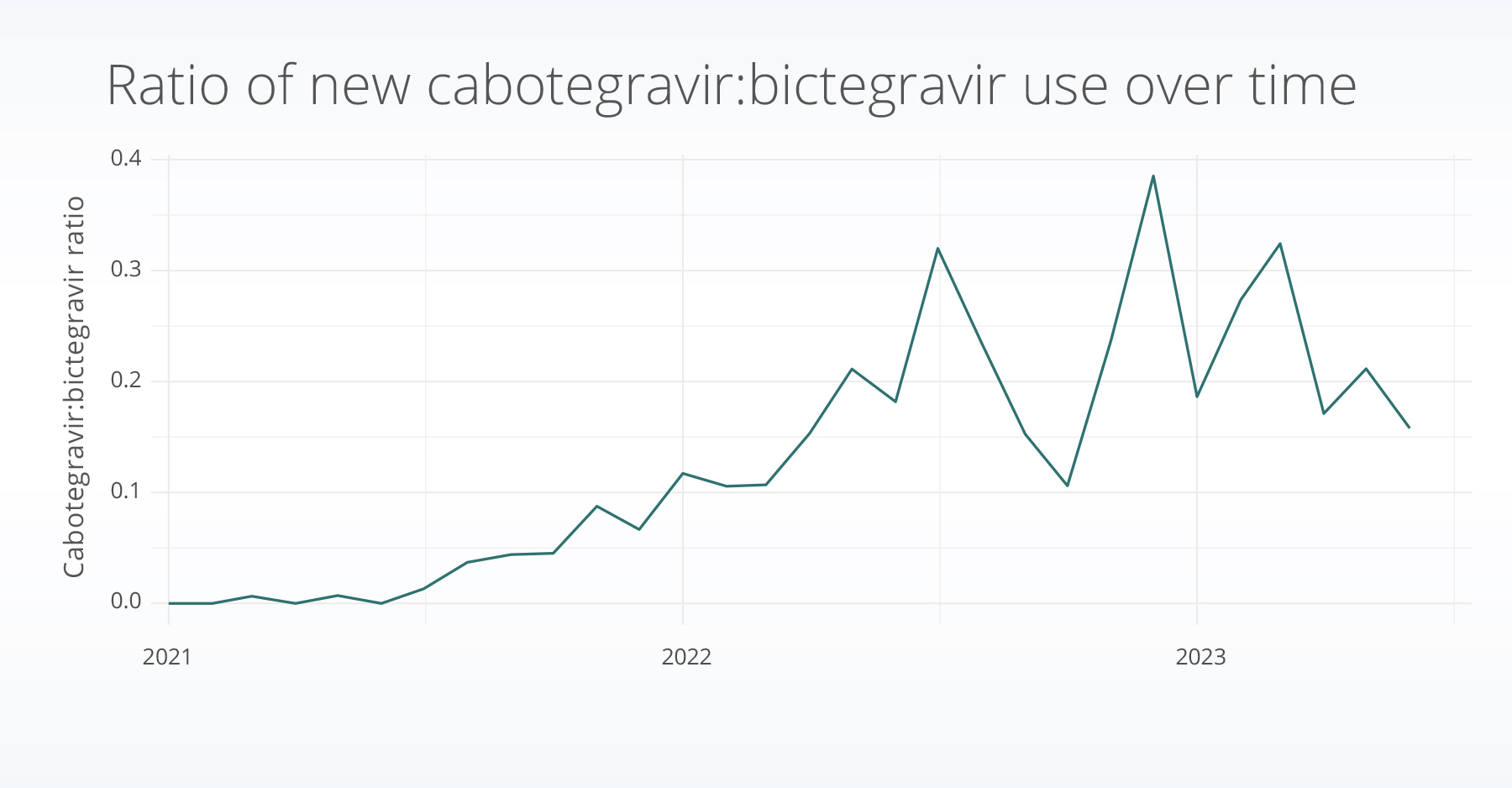
Discussion
In 2021 the FDA approved the first long-lasting injectable treatment for HIV. Although this may be a more convenient way to adhere to HIV treatments, our data is showing low uptake compared to a traditional oral prescription. Further, among the people who are taking cabotegravir, they have a higher individual income and education status, potentially indicating that access to cabotegravir may be a challenge.
In 2021, one group in the southern US implemented a pilot to administer long-acting injectable cabotegravir. They highlight additional challenges, such as insurance denials and substantial human resources to attain the drug, administer the drug, and support patients (Collins et al., 2022).
Cabotegravir was also approved for pre-exposure prophalixis (PrEP) in December 2021 (US Food & Drug Administration, 2021b). This is used in people without HIV, but who are at risk for exposure and contracting the virus. Recent data released from the CDC indicates that between 2019 and March 2023, white people with PrEP indications consistently received PrEP (all approved forms) at higher rates (60.1 – 94.0%) compared to Black/African American people (8.0 – 12.8%) and Hispanic or Latino individuals (14.6 – 24.4%) (Centers for Disease Control and Prevention, 2023b). In conjunction with our data – these data highlight disparities in access to both HIV treatments and PrEP (Sharfstein et al., 2022).
As with any study, there are a few limitations with this study. First as mentioned in the discussion, cabotegravir is approved for both treatment of people with HIV and for PrEP. We only studied its use as a treatment, not PrEP. We also classified patients according to the first drug prescribed or administered during the study period. Some patients may have switched drugs after their first prescription. Future studies are needed to assess the patient characteristics and timing of medication switches. Patients prescribed bictegravir likely have a higher rate of non-initiation (prescription was written by a physician, but never filled or initiated) than patients prescribed cabotegravir. Since cabotegravir is an injectable medication and administered in the physician’s office, gaps in initiation for cabotegravir are unlikely. Finally, we measured duration of HIV as the time between the first diagnosis within a Truveta healthcare system and the time of the first prescription. A person could have a diagnosis prior to coming to a Truveta health system, and thus the calculation for duration of time with HIV may be under-counting the actual time with HIV.
Many factors play into consistent medication adherence; however, this regular adherence is especially important for people with HIV. When the virus is undetectable in the blood, it is untransmittable. Thus, adequate treatments are crucial in decreasing the spread of HIV. Further research is needed to continue to understand uptake in new long-lasting injectable drugs and reduce potential disparities in access.
These are preliminary research findings and not peer reviewed. Data are constantly changing and updating. These findings are consistent with data accessed on June 21, 2023.
Citations
Centers for Disease Control and Prevention. (2021, May 20). Living with HIV. https://www.cdc.gov/hiv/basics/livingwithhiv/understanding-care.html
Centers for Disease Control and Prevention. (2023a, May 22). HIV Basics. https://www.cdc.gov/hiv/basics/statistics.html
Centers for Disease Control and Prevention. (2023b, October). Core Indicators for Monitoring the Ending the HIV Epidemic Initiative (Preliminary Data): National HIV Surveillance System Data Reported through June 2023; and Preexposure Prophylaxis (PrEP) Data Reported through March 2023. HIV Surveillance Data Tables. https://www.cdc.gov/hiv/library/reports/surveillance-data-tables/vol-4-no-3/index.html
Collins, L. F., Corbin-Johnson, D., Asrat, M., Morton, Z. P., Dance, K., Condra, A., Jenkins, K., Todd-Turner, M., Sumitani, J., Smith, B. L., Armstrong, W. S., & Colasanti, J. A. (2022). Early Experience Implementing Long-Acting Injectable Cabotegravir/Rilpivirine for Human Immunodeficiency Virus-1 Treatment at a Ryan White-Funded Clinic in the US South. Open Forum Infectious Diseases, 9(9), ofac455. https://doi.org/10.1093/ofid/ofac455
National Institute on Allergy and Infectious Diseases. (2019, May 21). HIV Undetectable=Untransmittable (U=U), or Treatment as Prevention. HIV/AIDS. https://www.niaid.nih.gov/diseases-conditions/treatment-prevention
Sharfstein, J. M., Killelea, A., & Dangerfield, D. (2022). Long-Acting Cabotegravir for HIV Prevention: Issues of Access, Cost, and Equity. JAMA, 327(10), 921. https://doi.org/10.1001/jama.2022.0420
US Food & Drug Administration. (2021a, January 21). FDA Approves First Extended-Release, Injectable Drug Regimen for Adults Living with HIV. https://www.fda.gov/news-events/press-announcements/fda-approves-first-extended-release-injectable-drug-regimen-adults-living-hiv
US Food & Drug Administration. (2021b, December 20). FDA Approves First Injectable Treatment for HIV Pre-Exposure Prevention. https://www.fda.gov/news-events/press-announcements/fda-approves-first-injectable-treatment-hiv-pre-exposure-prevention
