Beginning in Fall 2022, the news media has reported extensively on the use of Glucagon-like peptide-1 receptor agonist (GLP-1s) for weight loss (Belluz, 2023). Physicians have taken to TikTok to promote GLP-1s for weight loss, and celebrities & influencers – such as Elon Musk, Rosie O’Donnell, and Dolores Catania – have publicly shared that they were prescribed and take GLP-1s (Bienasz, 2023; Nelson, 2023; Smith, 2023).
GLP-1s have been approved for treatment of type 2 diabetes mellitus (T2DM) since 2005 and have also demonstrated high efficacy for weight loss (Jastreboff et al., 2022; Weghuber et al., 2022; Wilding et al., 2021). The first and only GLP-1-based drug currently approved for weight loss, semaglutide (brand name Wegovy), has been available since 2021. The FDA has also fast-tracked an application for tirzepatide (Eli Lilly, 2022). Similar versions of both semaglutide (brand name Ozempic) and tirzepatide (brand name Mounjaro) are approved for type 2 diabetes mellitus.
The combination of surging demand and supply chain disruption led to months-long shortages in availability of tirzepatide (brand name Mounjaro approved for T2DM) and semaglutide (brand names Ozempic approved for T2DM and Wegovy approved for weight loss) (Fiercepharma, 2023; Reuters, 2023; Today, 2023). Shortages have been particularly impactful for patients with T2DM trying to get access to medications (Loftus, 2023).
We were curious to learn if there has been an increase in the frequency of new GLP-1 prescriptions and how often they were prescribed on-label (for people with the FDA-approved indication) vs. off-label (for people without the FDA-approved indication). We focused on brand-name Mounjaro, Ozempic, and Wegovy.
We found increases in first-time prescribing of these drugs over the last two years. The drugs approved for T2DM – Mounjaro and Ozempic – were mostly prescribed off-label: 56% of patients newly prescribed Mounjaro or Ozempic did not have T2DM. In contrast, the drug approved for weight-loss – Wegovy – was mostly used on-label: 81% of patients prescribed Wegovy had overweight or obesity diagnoses.
Methods
We looked at first-time GLP-1 prescriptions for a subset of Truveta Data since January 2020. For this study, we were interested in understanding on- and off-label drug prescribing patterns of newer GLP-1-based drugs approved for either T2DM or weight loss. To accomplish this, we restricted to prescriptions with brand-name Mounjaro, Ozempic, and Wegovy. Prescriptions with an RxNorm code corresponding to the pharmacologic or ingredient name only (e.g., tirzepatide, semaglutide) were excluded. Only one drug was counted per person; therefore, if a person switched between drugs, the second drug was not captured in this analysis.
We classified prescriptions for Mounjaro and Ozempic (approved for T2DM) as on-label if the patient had a history of T2DM and off-label otherwise. We classified prescriptions of Wegovy (approved for overweight and obesity) as on-label if the patient had a history of BMI ≥27 or documentation of obesity, and off-label otherwise.
We summarized the number of new GLP-1 prescriptions over time, then stratified by on- vs. off-label use and brand. To normalize the data and remove the impact of changes in prescribing volumes for all drugs, we divided by the total number of prescriptions in Truveta Data each month. Prescribing volumes are presented as number of first-time GLP-1 prescriptions for each drug per 100,000 prescriptions.
Results
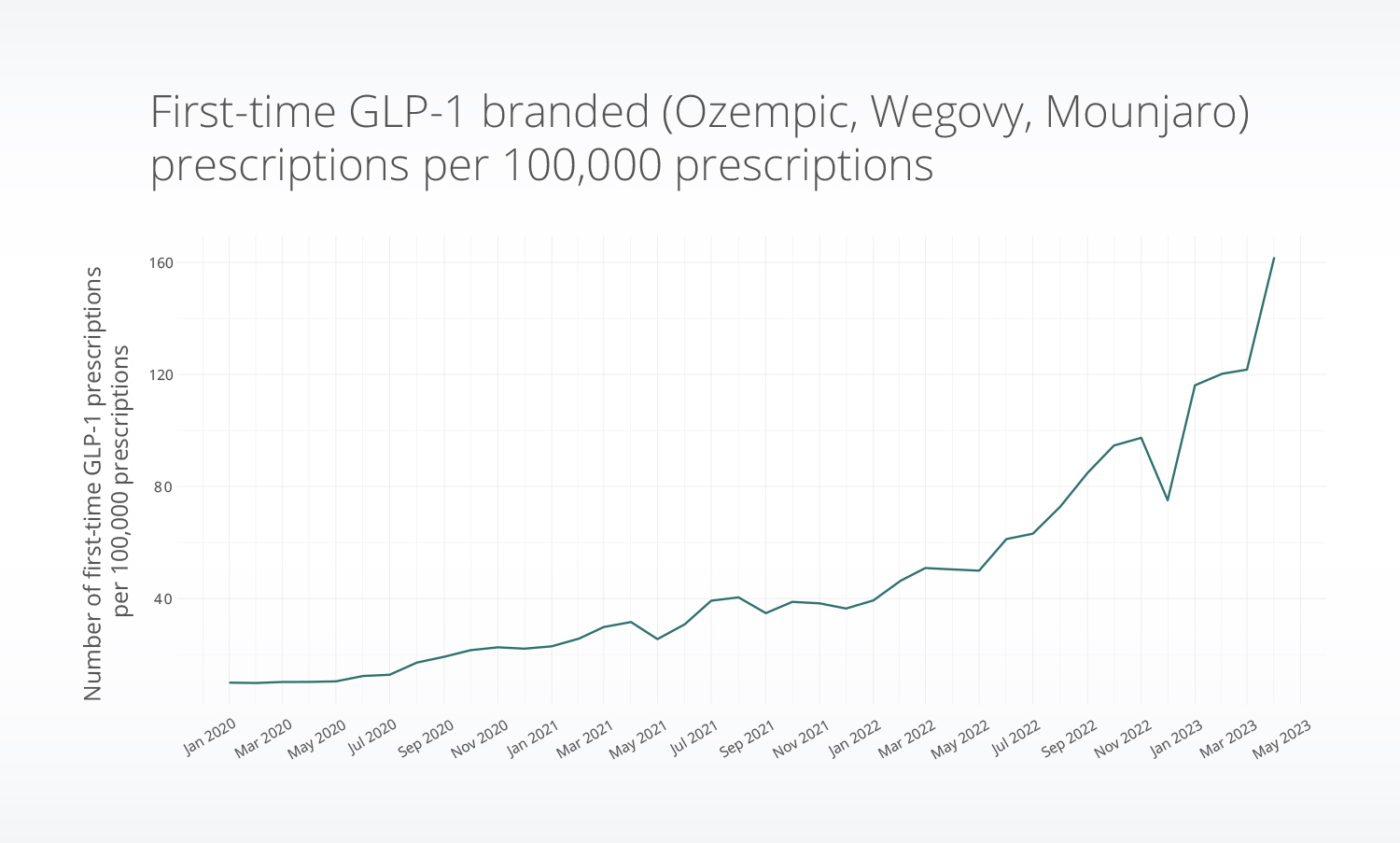
Over 120,000 patients received a first-time GLP-1 prescription for either Mounjaro, Ozempic, or Wegovy between January 2020 and April 2023. Ozempic was the only drug available for the full time period studied and had the highest prescriptions overall.
Overall, 50% of patients had evidence of the FDA-approved indication in their medical record (on-label use). However, on- vs. off-label prescribing differed between the drugs approved for T2DM (Ozempic and Mounjaro) and those approved for weight loss (Wegovy). The majority (56%) of patients prescribed the GLP-1 drugs approved for T2DM did not have evidence of T2DM in their medical record. In contrast, most patients (81%) prescribed GLP-1 approved for weight loss (Wegovy) had overweight or obesity indications.
Although the majority of first-time prescriptions occurred in the 45-64 age group, a higher proportion of patients with off-label prescriptions were female (off-label: 69%, on-label: 61%) and between 18-25 (off-label: 30%, on-label: 22%).
Overall changes over time
First-time prescriptions for GLP-1s increased since 2020, with marked changes in late 2022 and early 2023. The rate of first-time GLP-1 prescriptions for Ozempic, Wegovy, and Mounjaro increased by nearly 2.5x in March 2023 compared with March 2022.
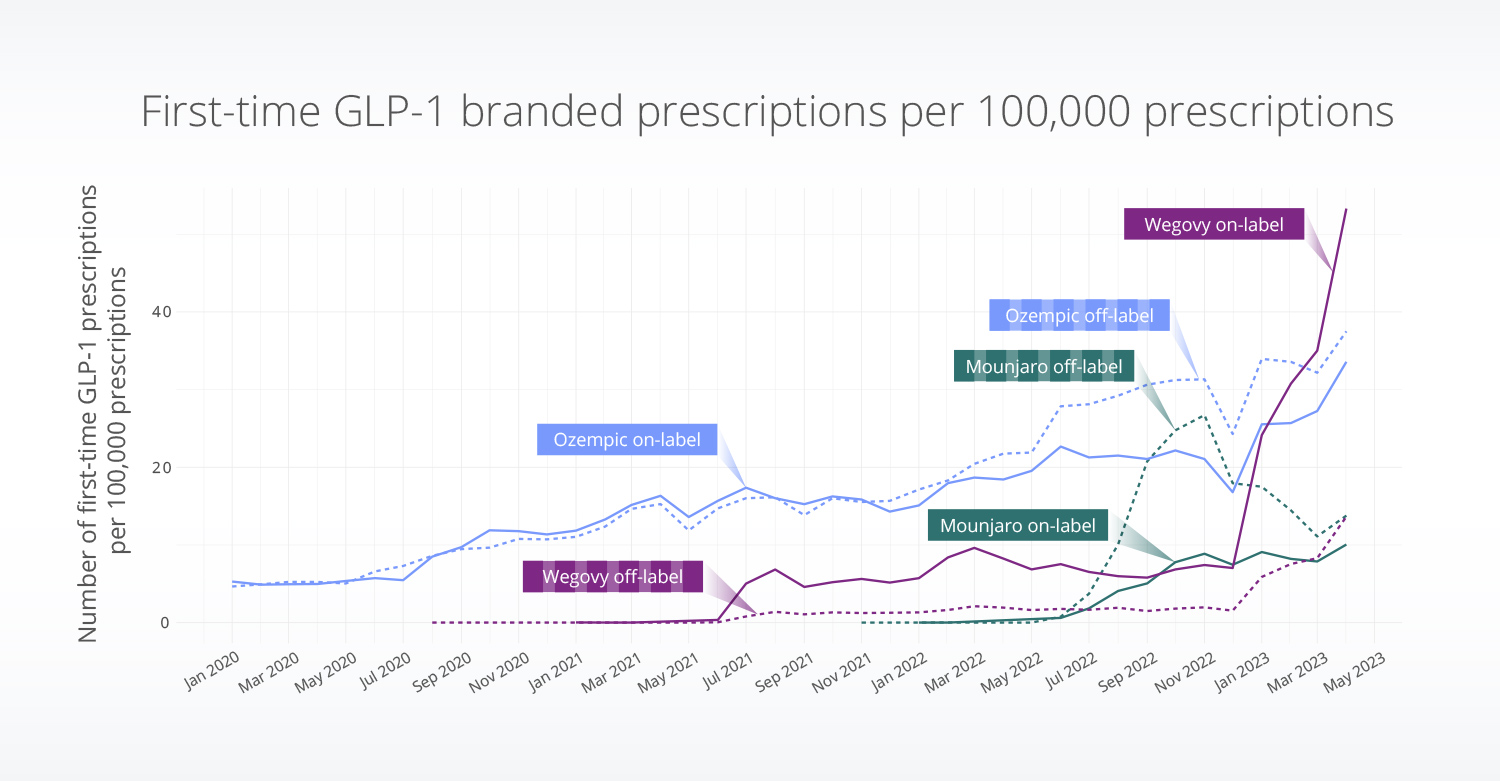
On- vs. off-label use
We observed prescribing trends by brand that aligned with reported shortages in brand availability. Wegovy (approved for weight loss) experienced months-long shortages that ended in December 2022, at which point first-time on-label prescribing increased sharply. At the same time, we observed a steep decrease in first-time off-label prescribing of Mounjaro. This may suggest that patients initiated Mounjaro off-label for overweight or obesity as an alternative to initiating Wegovy on-label due to Wegovy shortages. Shortages in Mounjaro were also reported beginning in December 2022 (Chen, 2022), but have ended in recent weeks.
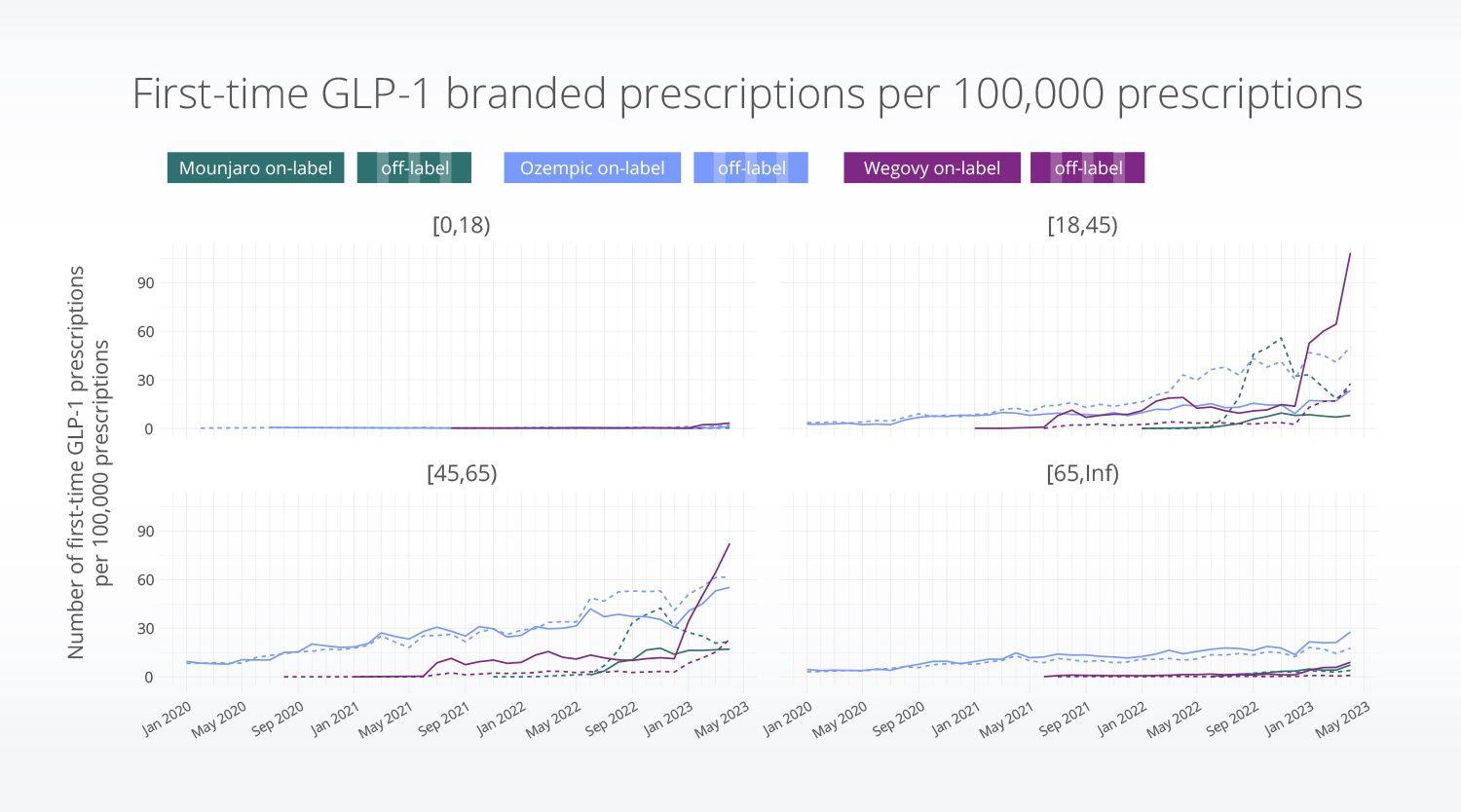
By age
Off-label use of Ozempic and Mounjaro was most common in the 18-45 and 45-65 age groups.
Discussion
Since 2020, these data suggest strong uptake of GLP-1-based drugs for overweight and obesity, including substantial off-label use of GLP-1 drugs that are approved for T2D only. We also observed changes in first-time prescribing volumes that corresponded closely to reported shortages. On-label prescribing of Wegovy (use among people with overweight or obesity) increased sharply in December 2022, after a months-long drug shortage of the drug ended (Today, 2023). Around the same time, off-label use of Mounjaro (use among people without T2D) sharply decreased, corresponding with both the end of the Wegovy shortage and the first report of Mounjaro shortages (which have now ended). This may suggest off-label prescribing of GLP-1s approved for T2D when GLP-1s approved for overweight and obesity are unavailable.
There are a few limitations associated with this study. First, we classified prescriptions as on- and off-label uses based on previous evidence in the patient’s record. If a patient was new to a health system where the medication was prescribed, they may be misclassified. We limited these data to patients’ first prescription in the GLP-1 drug class to capture changes in new demand for these drugs. Therefore, switches between prescriptions, such as a patient switching to Mounjaro when Wegovy became unavailable, are not captured in the current analysis. We also excluded GLP-1 data where the brand name did not exist. Finally, this analysis focused on prescribing changes only; we have not yet evaluated differences in dispensing patterns.
We are excited about this analysis, these data, and all the future questions they prompt for us to explore. Using up-to-date prescription and dispense data, we will continue to monitor uptake, treatment patterns, and outcomes of novel medications to treat obesity. We’re curious to dig into the medication history of people with T2DM and see if they switched brands when one became unavailable. We are also particularly interested in disproportionate access to these medications by social drivers of health, including treatment disruptions for patients with diabetes and gaps in access to highly effective treatments for overweight and obesity due to lack of insurance coverage (Wright et al., 2023). Finally, we will use Truveta’s weight, BMI, and cardiovascular event data to study the comparative effectiveness of these medications.
These are preliminary research findings and not peer reviewed. Data are constantly changing and updating. These findings are consistent with data pulled on April 10, 2023.
Citations
Belluz, J. (2023, February 7). Obesity in the age of Ozempic. Vox. https://www.vox.com/science-and-health/23584679/ozempic-wegovy-semaglutide-weight-loss-obesity
Bienasz, G. (2023, March 3). TikTok Docs Boosting Views and Profits with Weight Loss Drugs. Entrepreneur. https://www.entrepreneur.com/business-news/tiktok-docs-boosting-views-and-profits-with-weight-loss/446905
Chen, E. (2022, December 17). Eli Lilly diabetes drug Mounjaro in short supply, FDA says.STAT News. https://www.statnews.com/2022/12/17/eli-lilly-diabetes-drug-mounjaro-in-short-supply-fda-says/
Eli Lilly. (2022, October 6). Lilly receives U.S. FDA Fast Track designation for tirzepatide for the treatment of adults with obesity, or overweight with weight-related comorbidities | Eli Lilly and Company. https://investor.lilly.com/news-releases/news-release-details/lilly-receives-us-fda-fast-track-designation-tirzepatide
Liu, Angus. (2023, February 22). Lilly’s Mounjaro back in stock amid heightened obesity interest. FiercePharma. https://www.fiercepharma.com/pharma/eli-lilly-resolving-mounjaro-shortage-clearing-backlog-diabetes-drug-amid-weight-loss
Jastreboff, A. M., Aronne, L. J., Ahmad, N. N., Wharton, S., Connery, L., Alves, B., Kiyosue, A., Zhang, S., Liu, B., Bunck, M. C., & Stefanski, A. (2022). Tirzepatide Once Weekly for the Treatment of Obesity. New England Journal of Medicine, 387(3), 205–216. https://doi.org/10.1056/NEJMoa2206038
Loftus, P. (2023, February 22). Ozempic Runs Low for Diabetes Patients as Weight-Loss Use Surges—WSJ.Wall Street Journal. https://www.wsj.com/articles/ozempic-runs-low-for-diabetes-patients-as-weight-loss-use-surges-286bf21a
Nelson, E. L. (2023, April 7). 8 Celebrities Who’ve Admitted to Using Ozempic. Best Life. https://bestlifeonline.com/celebrities-who-took-ozempic/
Reuters. (2023, March 17). Novo Nordisk’s diabetes drug Ozempic back in supply in US after months of shortage. Reuters. https://www.reuters.com/business/healthcare-pharmaceuticals/novo-nordisks-diabetes-drug-ozempic-back-supply-after-months-shortage-2023-03-17/
Smith, R. (2023, January 18). Celebrities who have spoken out about Hollywood weight loss drug Ozempic. Newsweek. https://www.newsweek.com/celebrities-hollywood-weight-loss-diabetes-drug-ozempic-1774677
Today. (2023, January 5). Shortage of blockbuster anti-obesity drug ends: What to know about Wegovy. TODAY.com. https://www.today.com/health/diet-fitness/wegovy-weight-loss-shortage-ends-rcna64220
Weghuber, D., Barrett, T., Barrientos-Pérez, M., Gies, I., Hesse, D., Jeppesen, O. K., Kelly, A. S., Mastrandrea, L. D., Sørrig, R., & Arslanian, S. (2022). Once-Weekly Semaglutide in Adolescents with Obesity. New England Journal of Medicine, NEJMoa2208601. https://doi.org/10.1056/NEJMoa2208601
Wilding, J. P. H., Batterham, R. L., Calanna, S., Davies, M., Van Gaal, L. F., Lingvay, I., McGowan, B. M., Rosenstock, J., Tran, M. T. D., Wadden, T. A., Wharton, S., Yokote, K., Zeuthen, N., & Kushner, R. F. (2021). Once-Weekly Semaglutide in Adults with Overweight or Obesity. New England Journal of Medicine, 384(11), 989–1002. https://doi.org/10.1056/NEJMoa2032183
