- We explored the demographic characteristics of patients with type 2 diabetes mellitus, heart failure, and chronic kidney disease who received a prescription for empagliflozin (Jardiance) and/or dapagliflozin (Farxiga), two commonly used SGLT2i drugs.
- We saw differences in the rate of prescriptions for both drugs across demographic and disease groups. We also see an increase in the rate of dapagliflozin prescriptions for people with heart failure in May 2023, the month it was approved for patients with heart failure (regardless of ejection fraction). The ability to use near-real-time monitoring before and after drug approvals can yield new insights into current use patterns across the United States.
SGLT2i drugs work by inhibiting the sodium-glucose cotransporter-2 protein in the renal tubules, resulting in increased urinary glucose excretion and subsequent reduction in blood glucose levels3,4. In addition to demonstrated efficacy in improving glycemic control, reducing body weight, and lowering blood pressure, recent clinical trials have shown that SGLT2i have additional benefits in reducing the risk of cardiovascular events5 and slowing the progression of kidney disease4,6.
However, while SGLT2i share a similar mechanism of action, individual drugs within this class may have distinct characteristics that could influence their real-world usage and patient profiles across different indications7. Empagliflozin (Jardiance) and dapagliflozin (Farxiga) have also received FDA approval for patients with different diseases at different times (shown in Table 1 below)8,9. Understanding the demographics and uptake patterns of specific SGLT2i is crucial for optimizing treatment strategies and tailoring interventions to better meet the needs of patients with T2DM, heart failure, and chronic kidney disease (CKD). Therefore, in this insight, we aim to compare the demographics and prescribing patterns of two commonly prescribed SGLT2i: empagliflozin and dapagliflozin.
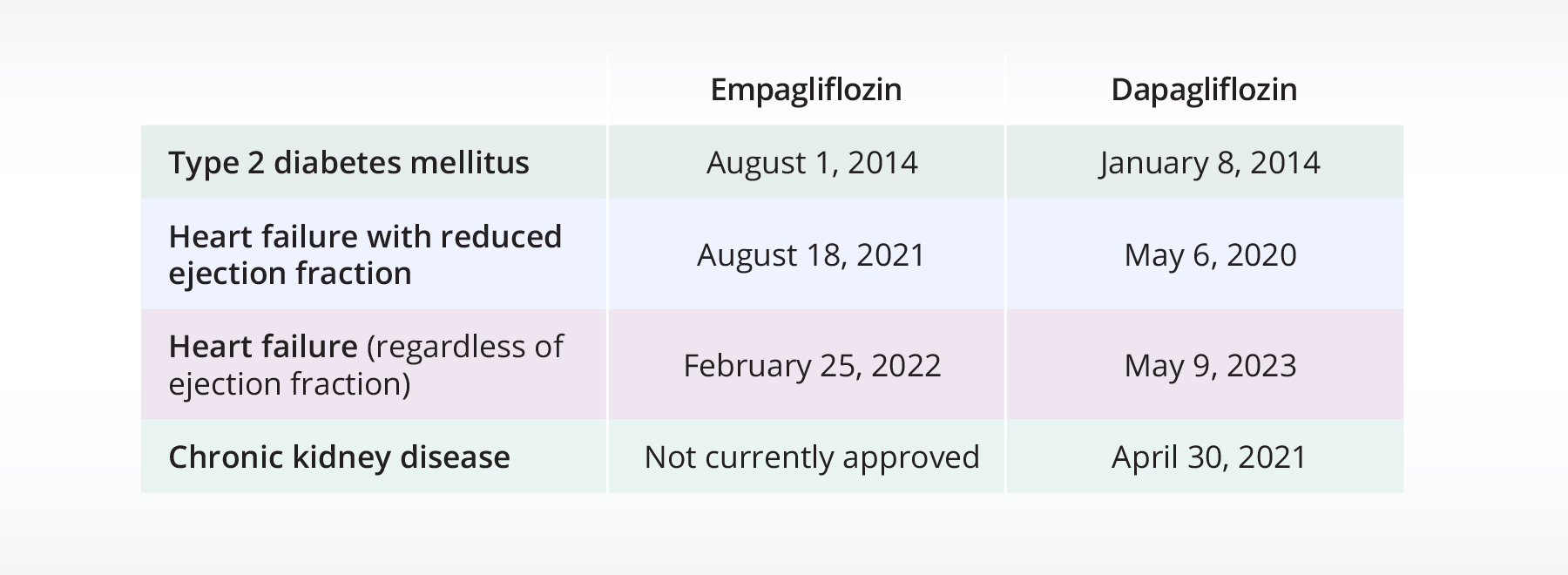
Table 1: FDA approval dates for empagliflozin and dapagliflozin for specific disease areas.
Methods
Both drugs were approved for heart failure with reduced ejection fraction (HFrEF) before approval for heart failure (regardless of ejection fraction). Therefore, in this study we separated the heart failure population into two populations: HFrEF and heart failure with preserved ejection fraction (HFpEF). We also included a population of people with heart failure that was unspecified specified. Additionally, dapagliflozin has been approved for CKD; however, it has not been approved for empagliflozin, therefore a CKD population for empagliflozin was not included.
For each drug, we looked at five populations:
- T2DM without heart failure or CKD
- HFrEF without T2DM or CKD
- HFpEF without T2DM or CKD
- Unspecified heart failure without T2DM or CKD
- CKD without T2DM or heart failure
We looked at the monthly rate of first-time prescriptions for each drug, per patients within each population who had not previously been prescribed the drug. We also describe the demographics of the populations on each drug. We included data through the end of May 2023.
Results
Overall, 10% of patients received first-time empagliflozin and/or dapagliflozin prescriptions during the study period. Over 300,000 people received first-time prescriptions for empagliflozin, over 125,000 people received first-time prescriptions for dapagliflozin, and over 41,000 people received both drugs at some point. People with T2DM received the largest percentage of both drugs. People who had T2DM and heart failure received empagliflozin at the highest rate (11%). Additionally, people who had T2DM and CKD or all three conditions (T2DM, CKD, and heart failure) also received empagliflozin at higher rates (9%). Dapagliflozin was highest amongst the same populations, but at lower rates (~3-4%).
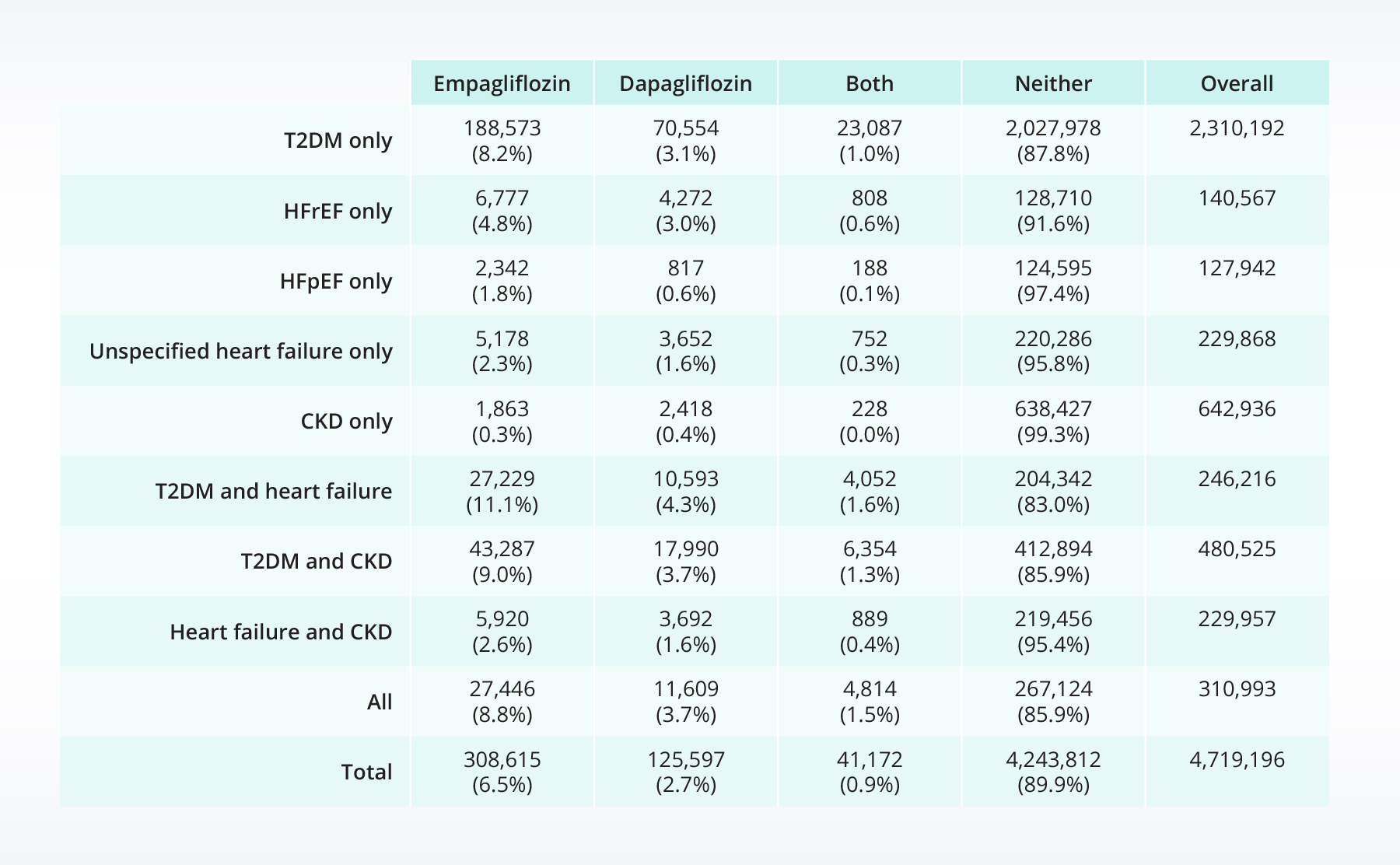
Table 2: Population of patients receiving first-time prescriptions for empagliflozin, dapagliflozin, both, or neither by condition.
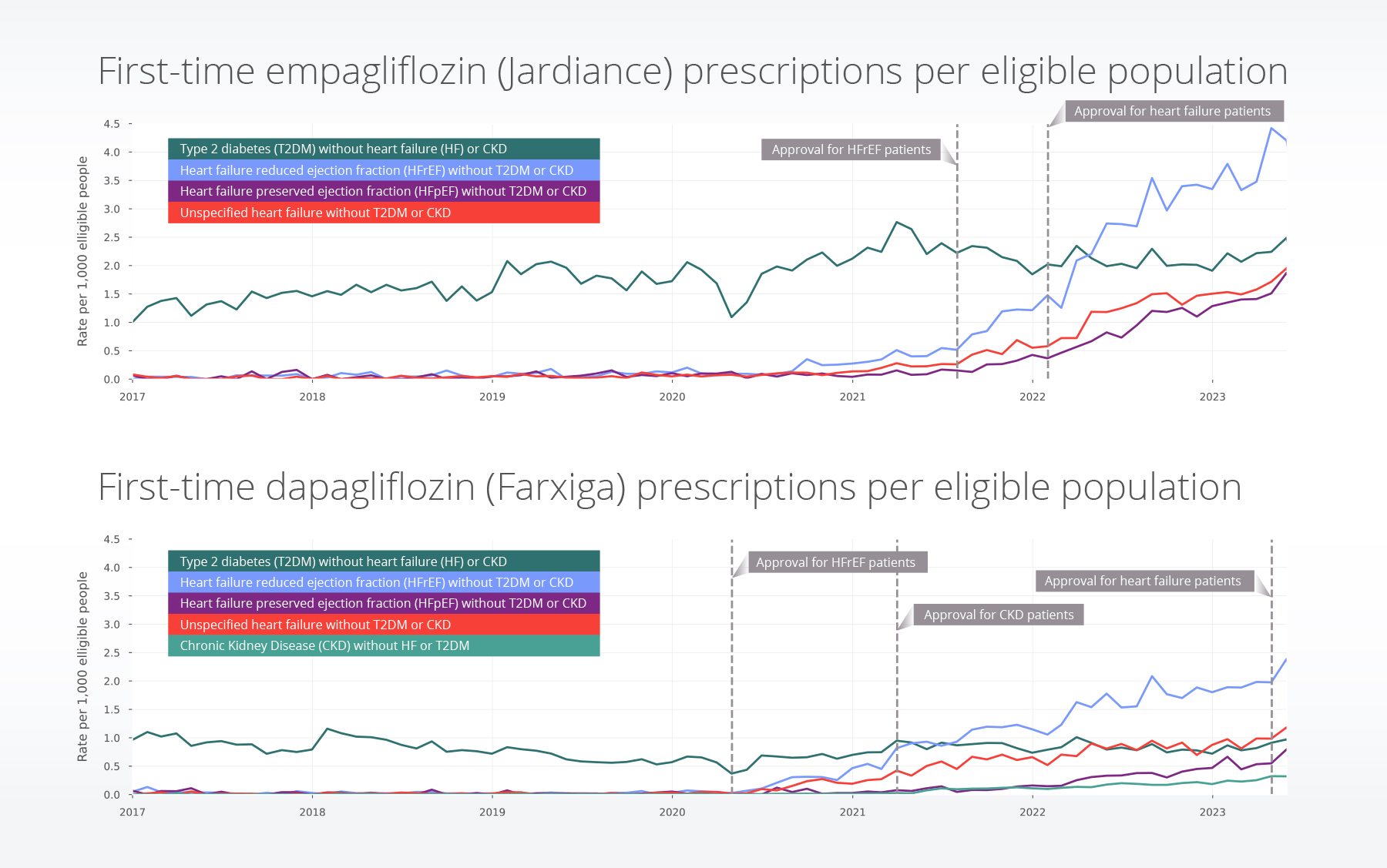
First-time prescriptions
Heart failure: We see notable increases in the dapagliflozin population who have heart failure (regardless of type) after its approval for HFrEF. We also see notable increases empagliflozin during this time period, potentially suggesting off-label prescribing. Although first-time prescription rates for empagliflozin are higher for the heart failure populations, with the recent approval of dapagliflozin on May 9, 2023 for people with heart failure (regardless of type), we already see increases in first-time prescribing trends for dapagliflozin during May 2023.
CKD: We see low rates of first-time dapagliflozin prescribed in the CKD-only population, even after the FDA approval in 2021. It’s important to note that this population excludes people who have CKD and T2DM or heart failure; therefore, there may have been an increase in the population who has CKD and additional comorbidities.
Demographics
T2DM: Descriptively men make up a slightly larger percentage of the population who received either dapagliflozin, empagliflozin, or both drugs. The 45–65-year-old population made up the largest age groups with prescriptions for empagliflozin and/or dapagliflozin. Of those prescribed at least one of the drugs, across demographic groups empagliflozin was taken at a higher percentage than dapagliflozin (shown in Table 3 below).
HFrEF: The population receiving either dapagliflozin, empagliflozin, or both are primarily male (>67%). Black or African American individuals make up a larger percentage of the individuals treated with these drugs (18-22%) compared with the percentage of the population who did not receive either drug (12.4%). The percentage of individuals receiving empagliflozin is higher than those receiving dapagliflozin, however the rates are closer than for the T2DM population (shown in the table below).
HFpEF: The absolute number of people with HFpEF receiving empagliflozin is higher than dapagliflozin (this is expected with the recent FDA approval8). Continued monitoring of these populations is warranted with the increase in recent dapagliflozin prescriptions during May 2023.
CKD: At the time of this blog, empagliflozin is not FDA approved for people with CKD. Therefore, the patients with CKD (without heart failure or T2DM) prescribed empagliflozin were prescribed it off-label.
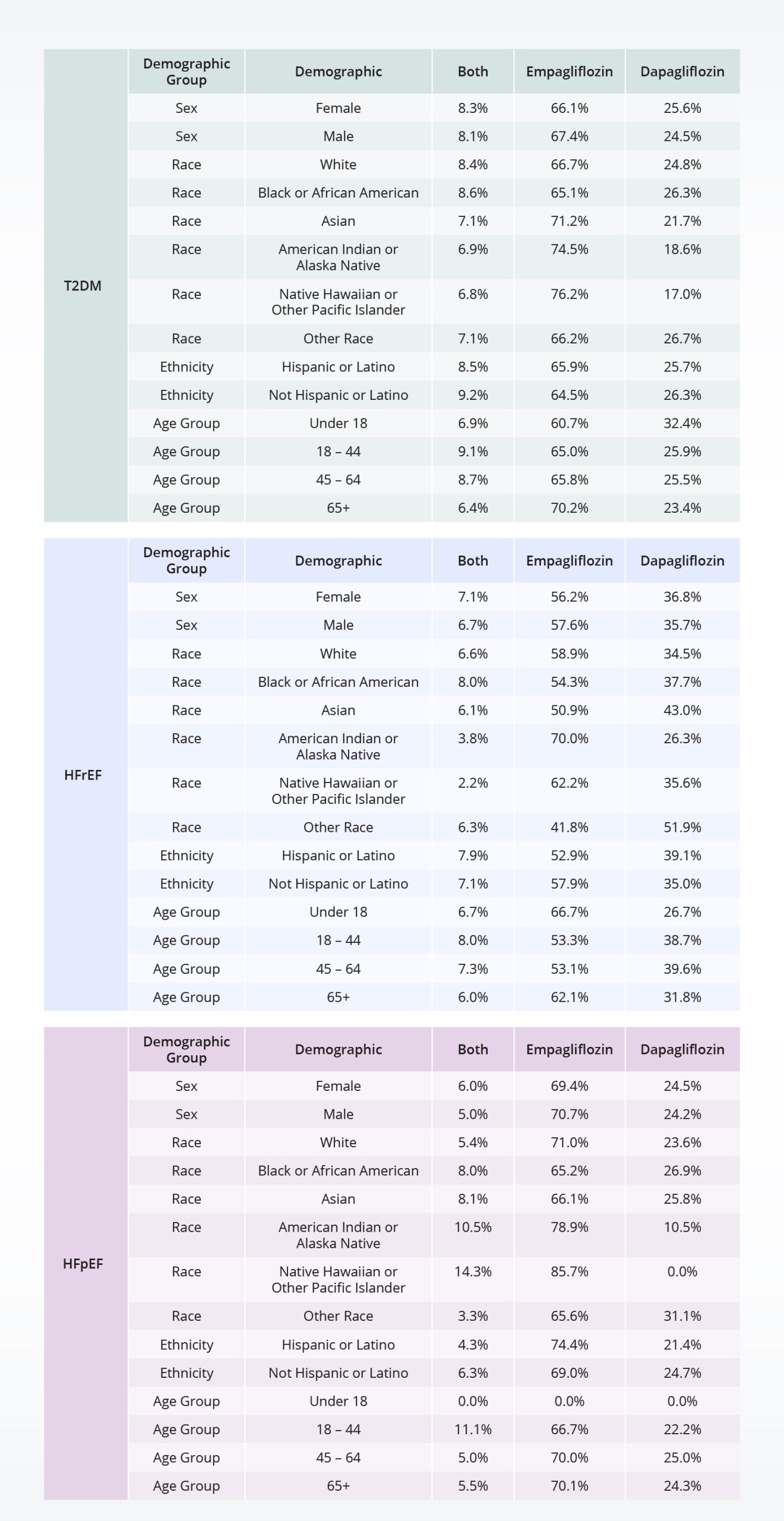
Table 3: Distribution rates of prescriptions for people who received empagliflozin and/or dapagliflozin, by disease and demographic groups.
Discussion
Among the populations analyzed, individuals with T2DM (with or without other comorbidities) received the highest percentage of both drugs, with the highest rate of empagliflozin prescriptions observed in patients with T2DM and heart failure. Dapagliflozin had smaller rates of prescriptions within the same populations (individuals with T2DM with and without comorbidities).
We saw differences in prescription rates across demographic and disease groups. In the T2DM and HFrEF populations, 45-65-year-old age group represented the largest population receiving empagliflozin and/or dapagliflozin prescriptions, whereas the HFpEF and CKD groups had a larger percentage of people in the over 65-age-group receiving prescriptions. For the CKD population, empagliflozin was prescribed off-label since it had not been FDA approved for CKD, while dapagliflozin had received approval for this indication. Continuous monitoring of the HFpEF (and unspecified heart failure) population will be interesting and will yield new trends with the recent dapagliflozin FDA approval for this group.
These findings have important implications for clinical practice, health policy planning, resource allocation, and identifying potential gaps in care. Understanding the demographic characteristics of patients receiving different SGLT2i is crucial for optimizing treatment strategies and tailoring interventions to better meet the needs of patients with T2DM, heart failure, and CKD. The higher prescribing rate of empagliflozin in the T2DM population suggests a preference for this medication in managing diabetes, potentially due to its longer availability in the market compared to dapagliflozin or coverage/price by an individual’s health plan. The differences in demographics across the two drugs may indicate variations in the prescribing patterns influenced by factors like efficacy, safety, or patient preferences.
It is worth noting that the study had certain limitations. First, we did not account for disease severity across any condition categories. People are likely to receive different treatments for a variety of reasons, and we did not take these into account. Further, there are multiple other SGLT2i drugs; however, they were not included in this analysis. Additionally, this study focused on prescriptions and did not account for changes in medication regimens, adherence rates, or treatment duration. There are contraindicators for both drugs; however, we did not exclude people from the population if they were prescribed contraindicated drugs or had contraindicated conditions. Finally, we did not account for regular care-seeking behavior in this analysis; this would indicate if the person were regularly seeking care from these healthcare organizations or if they received one prescription but received regular care a different healthcare system.
In conclusion, this study provides valuable insights into the demographics and prescribing patterns of empagliflozin and dapagliflozin in patients with T2DM, HFrEF, HFpEF, and CKD. The findings highlight the differences in patient profiles associated with each drug and emphasize the importance of considering these characteristics when making treatment decisions.
These are preliminary research findings and not peer reviewed. Data are constantly changing and updating. These findings are consistent with data accessed on June 29, 2023.
Citations
- Centers for Disease Control and Prevention. Type 2 Diabetes. Diabetes Basics. Published April 18, 2023. Accessed June 12, 2023. https://www.cdc.gov/diabetes/basics/type2.html
- Centers for Disease Control and Prevention. National and State Diabetes Trends. National and State Diabetes Trends. Published May 17, 2022. Accessed June 12, 2023. https://www.cdc.gov/diabetes/library/reports/reportcard/national-state-diabetes-trends.html
- Padda IS, Mahtani AU, Parmar M. Sodium-glucose transport protein 2 (SGLT2) inhibitors. In: StatPearls [Internet]. StatPearls Publishing; 2022.
- Ni L, Yuan C, Chen G, Zhang C, Wu X. SGLT2i: beyond the glucose-lowering effect. Cardiovasc Diabetol. 2020;19(1):98. doi:10.1186/s12933-020-01071-y
- Voors AA, Angermann CE, Teerlink JR, et al. The SGLT2 inhibitor empagliflozin in patients hospitalized for acute heart failure: a multinational randomized trial. Nat Med. 2022;28(3):568-574. doi:10.1038/s41591-021-01659-1
- Heerspink HJL, Karasik A, Thuresson M, et al. Kidney outcomes associated with use of SGLT2 inhibitors in real-world clinical practice (CVD-REAL 3): a multinational observational cohort study. The Lancet Diabetes & Endocrinology. 2020;8(1):27-35. doi:10.1016/S2213-8587(19)30384-5
- Monzo L, Ferrari I, Cicogna F, et al. Sodium-glucose co-transporter 2 inhibitors in heart failure: an updated evidence-based practical guidance for clinicians. European Heart Journal Supplements. 2023;25(Supplement_C):C309-C315. doi:10.1093/eurheartjsupp/suad055
- FDA. Drugs@FDA: FDA-Approved Drugs. https://www.accessdata.fda.gov/scripts/cder/daf/index.cfm?event=overview.process&ApplNo=204629
- FDA. Drugs@FDA: FDA-Approved Drugs. https://www.accessdata.fda.gov/scripts/cder/daf/index.cfm?event=overview.process&ApplNo=202293
