This blog is an extension of our poster presented at ISPOR, titled Real-World Effectiveness of SGLT2 Inhibitors Vs Metformin as First-Line Therapy in Type 2 Diabetes.
Individuals with type 2 diabetes mellitus (T2DM) have a higher risk of cardiovascular disease (Kannel & McGee, 1979; Savage, 1996). Lifestyle changes such as diet and exercise and medication can help patients achieve glycemic (blood sugar) control and reduce the risk of adverse cardiovascular outcomes (Holman et al., 2008).
Metformin is the recommended first-line antidiabetic medication for most patients who do not achieve glycemic control with lifestyle changes alone (American Diabetes Association, 2020; Buse et al., 2019; Davies et al., 2022). Metformin is a safe, effective, and inexpensive medication that has been used for over 30 years (Garber et al., 1997; UK Prospective Diabetes Study (UKPDS) Group., 1998). However, some newer therapies, such as a class of medications known as SGLT2i, have shown additional benefits for patients who are at higher risk of poor cardiovascular outcomes (Neal et al., 2017; Wiviott et al., 2019). Specifically, SGLT2i are recommended for patients with T2DM and either atherosclerotic cardiovascular disease (ASVCD), heart failure (HF), or chronic kidney disease (CKD).
While the benefits of SGLT2i for higher-risk populations have been shown through randomized clinical trials (RCTs), the benefits of initiation with SGLT2i in the broader population are not clear (Neal et al., 2017; Wiviott et al., 2019, 2019).
We studied the comparative effectiveness of T2DM treatment initiation with SGLT2i versus metformin in an all-comer population (i.e., all patients who initiated these medications in real-world clinical practice, regardless of indication). We evaluated how both cardiovascular and glycated hemoglobin (HbA1c) outcomes differ between patients starting treatment with SGLT2i or metformin in real-world clinical practice.
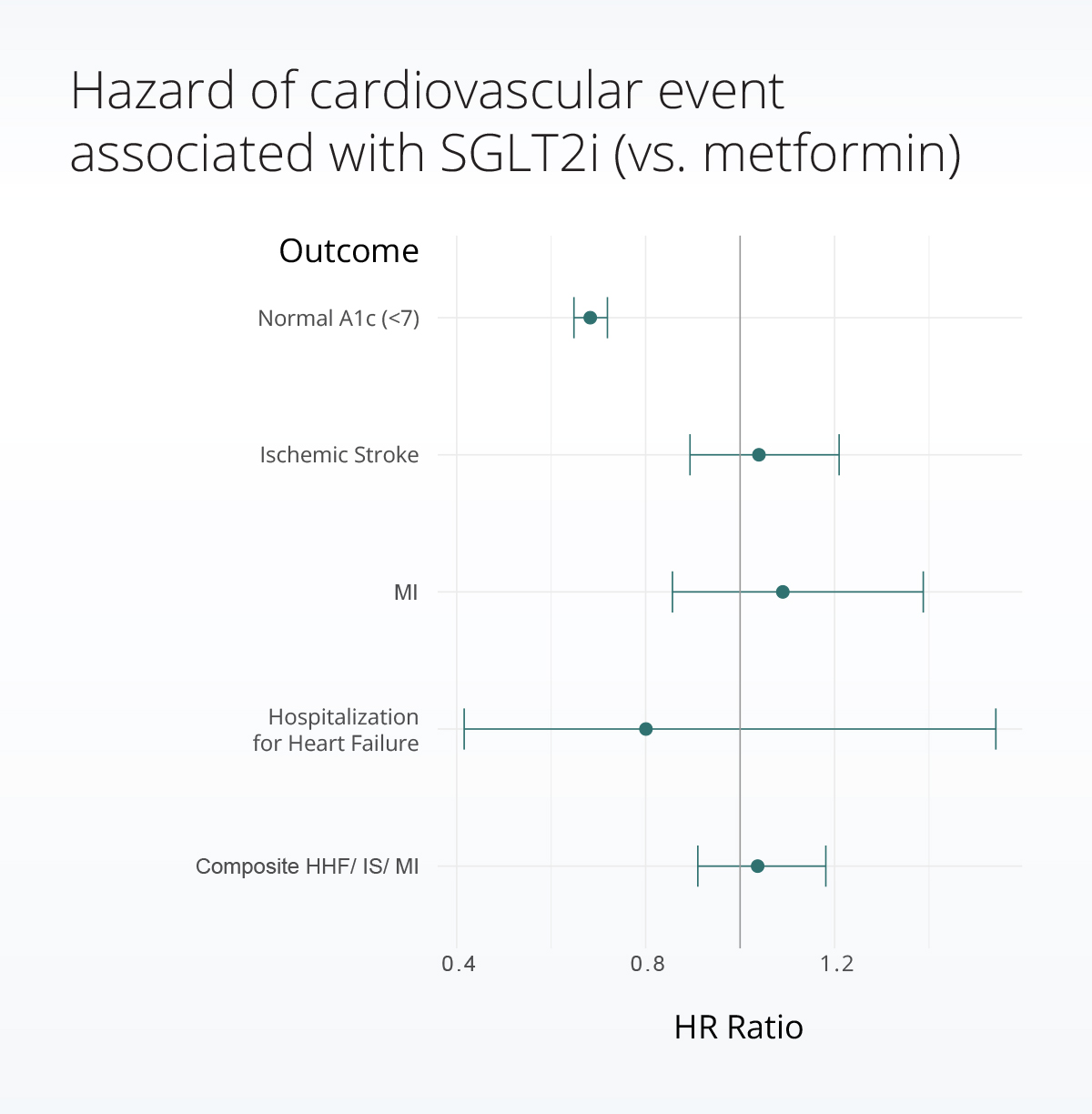
We found no significant difference in risk of cardiovascular outcomes between patients on metformin or SGLT2i, but we did find those starting SGLT2i experienced smaller reductions in blood sugar (HbA1c) and were less likely to reach a ‘normal’ level (HbA1c <7).
Methods
Population
Within a subset of the Truveta Data, we identified adult patients with at least one T2DM diagnosis who initiated treatment with either metformin or SGLT2i (canagliflozin, dapagliflozin, or empagliflozin) monotherapy between 2016 and 2022. We excluded patients who previously used an antidiabetic medication and those who started another antidiabetic medication at the same time. We also excluded patients with a history of gestational diabetes, organ transplant, and/or end-stage renal disease (ESRD), and those missing age or gender. Finally, to increase the reliability of follow-up data, we limited our population to patients who received regular care at a Truveta healthcare system: those with at least three usual care encounters in the three years prior to starting SGLT2i or metformin.
Follow-up
We followed patients for up to five years from the time they started the medication. We censored patients at the first of: treatment discontinuation, initiation of the comparator medication (e.g., a metformin user began taking an SGLT2i), or the end of follow-up (12/31/22). Censoring is used in time-to-event analysis to indicate the end of follow-up for a patient. A censoring date represents the last time a patient’s outcomes were observable. Censoring does not preclude the patient from experiencing the outcome, but the outcome would not be observed in the study. Despite having incomplete follow-up, these patients can still contribute valuable information to our study.
Cardiovascular outcomes
In keeping with previous studies, our primary outcome was occurrence of a composite cardiovascular event: myocardial infarction (MI), stroke, or hospitalization for heart failure (HHF). We also evaluated each cardiovascular event separately as secondary outcomes.
We compared the risk of cardiovascular events between those initiating SGLT2i vs. metformin using propensity score matched Cox proportional hazards models. Propensity scores allowed us to balance the metformin and SGLT2i populations, so that differences in outcomes were not attributable to underlying differences between patients prescribed metformin vs. SGLT2i, such as age and cardiovascular risk. Propensity scores accounted for demographics, T2DM duration and complications, comorbidities, cardiovascular risk factors, health care utilization factors, and calendar year of initiation. We used 1:1 nearest neighbor propensity score matching and included the same variables in the Cox proportional hazards model to minimize the risk of residual confounding.
HbA1c outcomes
Two approaches were taken to measure the difference in HbA1c outcomes.
First, among those with elevated HbA1c (>7) at baseline, we compared the time until reaching a normal (<7) HbA1c level between those started on SGLT2i vs metformin. We used a 1:1 propensity score matched Cox proportional hazards model. Because the population with elevated HbA1c is a subset of the full population, we performed propensity score matching separately for this population using the same process described above.
Second, we compared the magnitude of 12-month HbA1c changes between patients who started on SGLT2i versus metformin. Because our data comes from real-world clinical practice rather than a protocolized RCT, not all our patients had follow-up HbA1c measures exactly 12 months after starting medication. Therefore, we used the HbA1c value measured closest to 12 months after initiation, up to 6 months before and after. Only patients with baseline and follow-up values were included in this analysis. For this analysis, we used propensity score matched linear regression to compare the difference in 12-month HbA1c between patients started on SGLT2i versus metformin.
Results
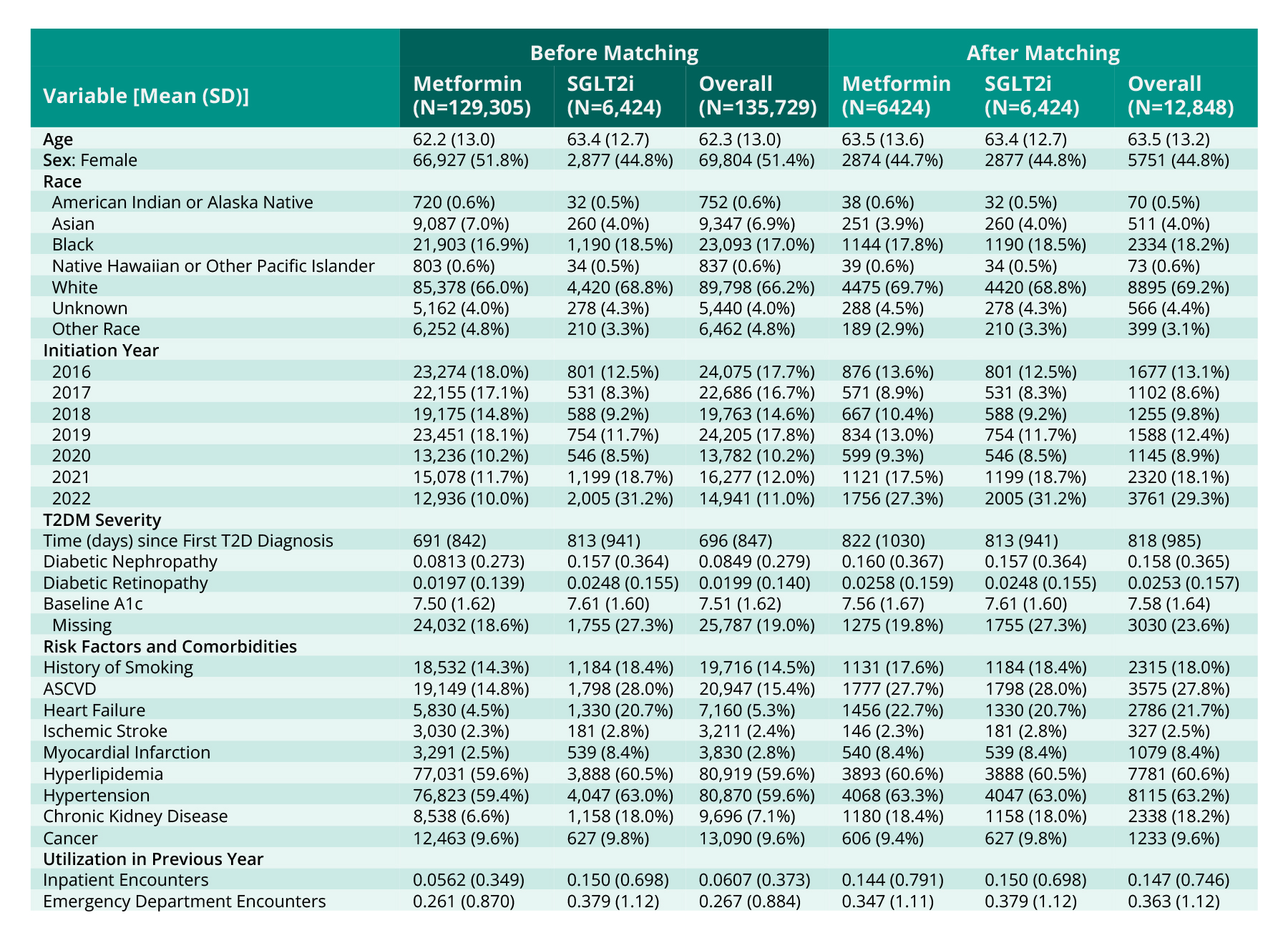
A total of 135,729 patients met our study criteria. As expected, most patients starting medication for T2DM initiated with metformin (95%), while 5% initiated with an SGLT2i. Overall, a higher proportion of those initiated on SGLT2i were male (55% on SGLT2i vs. 48% on metformin), white (69% on SGLT2i vs. 66% on metformin), or had initiated in 2020 or later (58% on SGLT2i vs. 32% on metformin). As expected, those who initiated with SGLT2i also had higher rates of ASCVD, heart failure, CKD, and other comorbidities at baseline.
After 1:1 propensity-score matching, N = 12,848 patients remained in our analytic sample. No SGLT2i patients were discarded. Propensity score matching achieved good balance (all standardized mean differences < 0.1 (Table 1)).
Discussion
In a real-world population, initiation of T2DM treatment with SGLT2i remained rare and most frequently occurred in patients with a greater number of comorbidities and an elevated risk of poor cardiovascular outcomes. Compared to similar patients initiated on metformin, patients initiated on SGLT2i had no difference in risk of composite cardiovascular events. However, patients initiated on SGLT2i experienced smaller reductions in HbA1c over 12 months and were less likely to achieve normal HbA1c levels (<7).
This study has a few limitations. First, previous claims-based studies have identified significant reductions in cardiovascular events for SGLT2i users (Fralick et al., 2021; Shin et al., 2022). The lack of significant differences in this study may be attributable to a comparatively smaller sample size. Despite a large population meeting our inclusion criteria, first-line treatment initiation with SGLT2i remains rare, which limited the size of our analyzable population. While the use of EHR data enabled us to leverage recent data and include changes in lab values (HbA1c) that were not previously studied, EHR data has some inherent limitations for comparative effectiveness studies. EHR data only captures clinical information for care received at included facilities. In our study, patients who previously received T2DM medications at other facilities would be misclassified as new users of treatment. Misclassification is likely to disproportionately impact the SGLT2i group because SGLT2i are less often used as a first-line treatment. Additionally, clinical outcomes would be missed if patients sought care elsewhere, such as going to a facility closer to their home when they experience symptoms of a myocardial infarction. The problem of uncaptured exposures and outcomes in EHR data can be mitigated by restricting the patient population to those who regularly use and/or are loyal to the system (Lin et al., 2018), as was done in our study. Finally, we did not include mortality as an outcome in this particular study.
These are preliminary research findings and not peer reviewed. Data are constantly changing and updating. These findings are consistent with data pulled January 9, 2023.
Citations
American Diabetes Association. (2020). 9. Pharmacologic Approaches to Glycemic Treatment: Standards of Medical Care in Diabetes—2021. Diabetes Care, 44(Supplement_1), S111–S124. https://doi.org/10.2337/dc21-S009
Buse, J. B., Wexler, D. J., Tsapas, A., Rossing, P., Mingrone, G., Mathieu, C., D’Alessio, D. A., & Davies, M. J. (2019). 2019 Update to: Management of Hyperglycemia in Type 2 Diabetes, 2018. A Consensus Report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care, 43(2), 487–493. https://doi.org/10.2337/dci19-0066
Davies, M. J., Aroda, V. R., Collins, B. S., Gabbay, R. A., Green, J., Maruthur, N. M., Rosas, S. E., Del Prato, S., Mathieu, C., Mingrone, G., Rossing, P., Tankova, T., Tsapas, A., & Buse, J. B. (2022). Management of Hyperglycemia in Type 2 Diabetes, 2022. A Consensus Report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care, 45(11), 2753–2786. https://doi.org/10.2337/dci22-0034
Fralick, M., Schneeweiss, S., Redelmeier, D. A., Razak, F., Gomes, T., & Patorno, E. (2021). Comparative effectiveness and safety of sodium-glucose cotransporter-2 inhibitors versus metformin in patients with type 2 diabetes: An observational study using data from routine care. Diabetes, Obesity and Metabolism, 23(10), 2320–2328. https://doi.org/10.1111/dom.14474
Garber, A. J., Duncan, T. G., Goodman, A. M., Mills, D. J., & Rohlf, J. L. (1997). Efficacy of Metformin in Type II Diabetes: Results of a Double-Blind, Placebo-controlled, Dose-Response Trial. The American Journal of Medicine, 103(6), 491–497. https://doi.org/10.1016/S0002-9343(97)00254-4
Holman, R. R., Paul, S. K., Bethel, M. A., Matthews, D. R., & Neil, H. A. W. (2008). 10-Year Follow-up of Intensive Glucose Control in Type 2 Diabetes. New England Journal of Medicine, 359(15), 1577–1589. https://doi.org/10.1056/NEJMoa0806470
Kannel, W. B., & McGee, D. L. (1979). Diabetes and cardiovascular risk factors: The Framingham study. Circulation, 59(1), 8–13. https://doi.org/10.1161/01.CIR.59.1.8
Lin, K. J., Singer, D. E., Glynn, R. J., Murphy, S. N., Lii, J., & Schneeweiss, S. (2018). Identifying patients with high data completeness to improve validity of comparative effectiveness research in electronic health records data. Clinical Pharmacology and Therapeutics, 103(5), 899–905. https://doi.org/10.1002/cpt.861
Neal, B., Perkovic, V., Mahaffey, K. W., de Zeeuw, D., Fulcher, G., Erondu, N., Shaw, W., Law, G., Desai, M., & Matthews, D. R. (2017). Canagliflozin and Cardiovascular and Renal Events in Type 2 Diabetes. New England Journal of Medicine, 377(7), 644–657. https://doi.org/10.1056/NEJMoa1611925
Savage, P. J. (1996). Cardiovascular Complications of Diabetes Mellitus: What We Know and What We Need To Know about Their Prevention. Annals of Internal Medicine, 124(1_Part_2), 123–126. https://doi.org/10.7326/0003-4819-124-1_Part_2-199601011-00008
Shin, H., Schneeweiss, S., Glynn, R. J., & Patorno, E. (2022). Cardiovascular Outcomes in Patients Initiating First-Line Treatment of Type 2 Diabetes With Sodium–Glucose Cotransporter-2 Inhibitors Versus Metformin. Annals of Internal Medicine, 175(7), 927–937. https://doi.org/10.7326/M21-4012
UK Prospective Diabetes Study (UKPDS) Group. (1998). Effect of intensive blood-glucose control with metformin on complications in overweight patients with type 2 diabetes (UKPDS 34). The Lancet, 352(9131), 854–865.
Wiviott, S. D., Raz, I., Bonaca, M. P., Mosenzon, O., Kato, E. T., Cahn, A., Silverman, M. G., Zelniker, T. A., Kuder, J. F., Murphy, S. A., Bhatt, D. L., Leiter, L. A., McGuire, D. K., Wilding, J. P. H., Ruff, C. T., Gause-Nilsson, I. A. M., Fredriksson, M., Johansson, P. A., Langkilde, A.-M., & Sabatine, M. S. (2019). Dapagliflozin and Cardiovascular Outcomes in Type 2 Diabetes. New England Journal of Medicine, 380(4), 347–357. https://doi.org/10.1056/NEJMoa1812389
