The pace of new therapy adoption has been limited by the ability to produce data that the medical community trusts. Clinical researchers have historically had difficulty accessing and cleaning healthcare data, wrestling with hard-to-use tools, and developing and maintaining their own bespoke analytics code. Collaborative efforts required manual oversight of access permissions, necessitating meticulous version tracking for any alterations made to the data, analysis code, and any study content by collaborators. Collectively, these hurdles have slowed the pace of learning in healthcare, limiting advancements in care delivery.
Truveta addresses these research challenges through Truveta Studio, which enables scientifically rigorous research with immediate access to data, powerful analytics and AI, and real-time collaboration. Truveta Studio enables access to and analysis of Truveta Data, regulatory-grade data from EHRs linked with claims and SDOH data. Truveta Studio is designed to support the ongoing process of scientific inquiry and research, with the latest enhancements improving how researchers create fit-for-purpose datasets, analyze large populations, train AI models, and collaborate for peer review. The new and enhanced features include:
Immediate access to data
Instant access to representative, patient-level, real-world data for any population of interest, with insight on the size of the population, the representativeness of the data benchmarked against the US census, and clinical quality metrics for the study population compared to all Truveta Data. Truveta Data may include detail on diagnoses, lab results, biometric data, imaging studies, device use, medication dispense, and more – enabling precise population definitions.
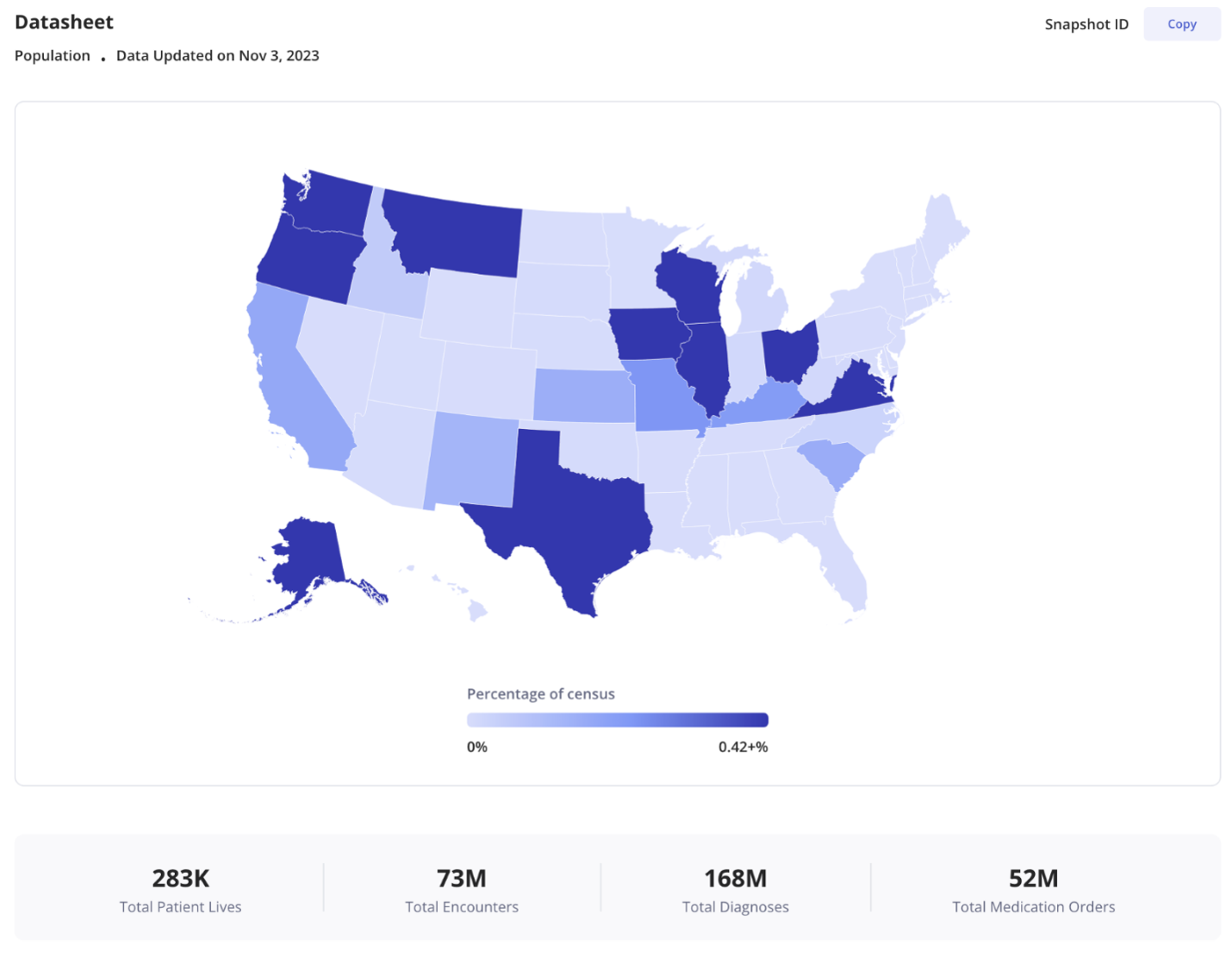
Fast expression of clinically meaningful populations. With Truveta Prose, a powerful and intuitive search language, researchers can define a study population, search an exabyte of Truveta Data, and compile a population dataset – with just one query. Researchers can use Prose to study daily updated patient journeys with unprecedented granularity, while protecting patient privacy with full regulatory compliance. Researchers can specify demographic, temporal or geographic criteria in Prose without exposure to row-level data.
The ability to iteratively build a patient population with real-time visualization of demographics. Researchers can dynamically refresh populations to test various permutations of inclusion/exclusion criteria to understand the resulting impact on the population and outcomes and to assess study feasibility. Additionally, researchers can define an index event as an anchor in each patient’s timeline, enabling them to compute comorbidities/outcomes relative to the index event. After defining an index event, an age visualization will appear in Truveta Studio, providing a clear and intuitive representation of age based on a defined index event.
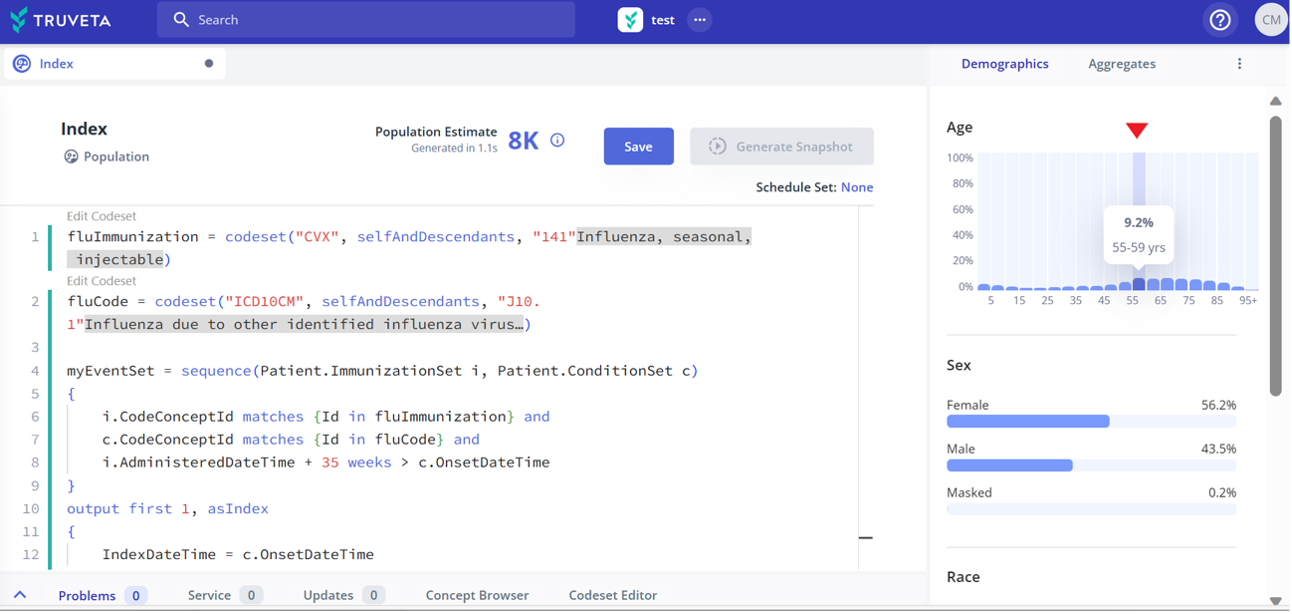
Powerful analytics and AI
Powerful analytics for analyzing large populations using pre-installed notebooks with the latest medical statistics and visualization libraries. Researchers in Truveta Studio now have access to a Spark-based notebook environment enabling iterative data exploration and analysis of large workloads. Truveta Studio now leverages Apache Spark as its underlying analytics engine. Spark is a foundational framework that empowers data scientists to efficiently process and analyze large-scale datasets within their notebook environments. It excels in handling massive workloads containing billions of patient data points within minutes. Researchers can work in their preferred language, or even mix-and-match various languages within the same notebook. Truveta Studio notebooks support Python, R, and Spark SQL languages.
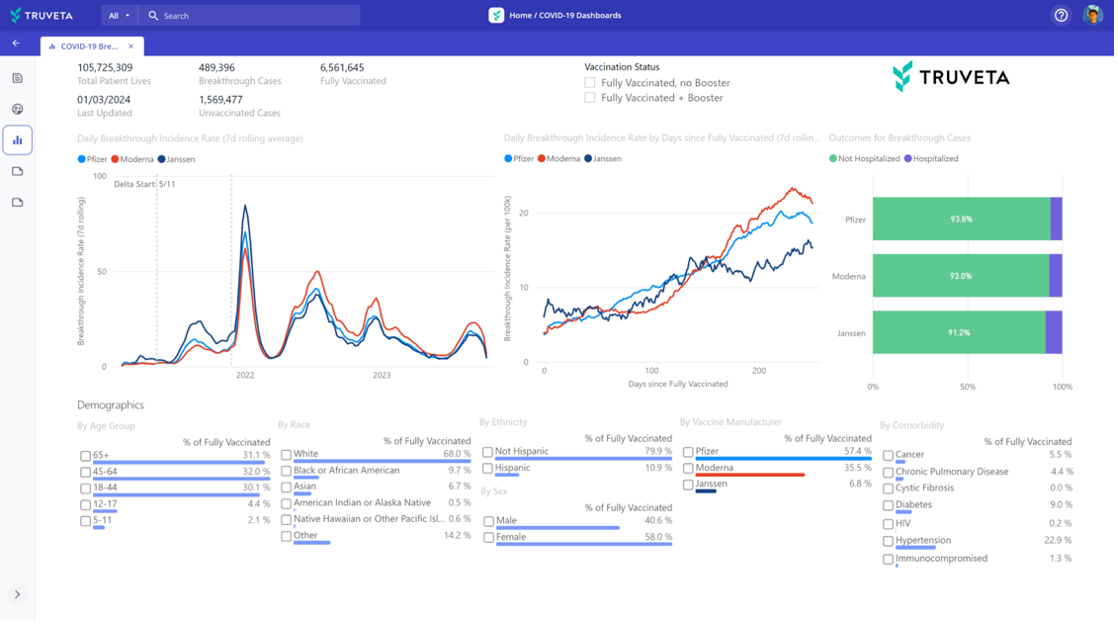
Daily updating dashboards and visualizations for safety monitoring or ongoing analyses. This is particularly helpful for time-sensitive research such as pharmacovigilance and epidemiological monitoring or ongoing analyses of targeted patient populations using real-world data. In the latest update, researchers can create Power BI dashboards within Truveta Studio based on their population snapshots. These dashboards can be scheduled to refresh daily, weekly, or monthly. Researchers can easily share a dashboard link with stakeholders for monitoring purposes.
The ability to develop, deploy, use, and share powerful AI models. These models can be developed on top of Truveta Data, a researcher’s own proprietary datasets, and rich public datasets such as Drug Central, PubMed, MeSH, Reactome, and DisGeNET. To build or fine-tune AI models, Truveta Studio provides a robust AI infrastructure, which includes generic toolsets including Kubeflow and PyTorch/TF, as well as specific generative frameworks like Nemo and Hugging Face Transformers to support state-of-the-art generative AI.
Real-time collaboration
Access to thousands of clinical data definitions, templates, and studies to build upon within Truveta Library. Truveta data definitions are easy to search for and reuse, simplifying the task of creating clinically meaningful populations from real-world data. The number of available data definitions is growing continuously, with hundreds of definitions newly added. Similarly, researchers can duplicate existing studies in Truveta Library to use as a starting point, making relevant changes and additions to suit their research and accelerate the research process. Several end-to-end studies have been added to Truveta Studio in recent months, showcasing new capabilities such as absolute-time analysis.
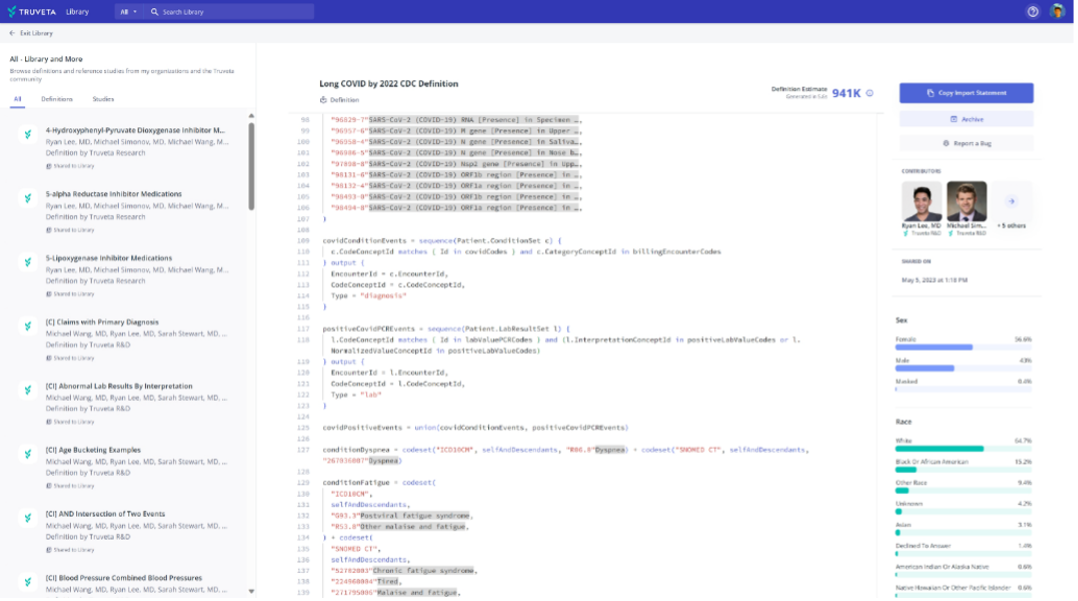
The ability to easily collaborate with others on study development, replication, and publication or regulatory filing. Truveta Studio not only enables researchers to collaborate with colleagues in their own organization, but also with researchers from other Truveta customer organizations. Additionally, Truveta Studio now supports workspaces, which allows for better organization across research teams within an organization. With workspaces, each team can have a dedicated environment to perform their studies.
Collectively, these features equip life science, government, and healthcare organizations with powerful real-world data analytics to support a broad range of scientifically rigorous research objectives, including:
- Safety monitoring: Fulfill post-market requirements more efficiently with regulatory-grade data.
- Comparative effectiveness: Establish product differentiation and increase market adoption with timely patient-level outcomes and SDOH data.
- Label expansion: Identify emerging opportunities to expand product usage and improve care.
- Clinical trials: Optimize clinical trial protocols through dynamic testing of inclusion and exclusion criteria and accelerate trial timelines by identifying and enrolling the right patients faster.
- Closing care gaps: Collaborate with health systems to advance adoption of insights into patient care.
As with all parts of Truveta, Truveta Studio benefits from continuous improvement. The Truveta Library is expanding each day — from new reference studies to increasingly advanced data definitions covering a diverse set of clinical subjects. Contributions and feedback from our community provide ongoing validation of these definitions and studies.
Truveta Studio is already being used by leading health systems such as Providence, Baylor Scott and White, and Novant Health; and innovative life science companies such as Boston Scientific, Boehringer Ingelheim, Reprieve Cardiovascular, and SK Life Science, to monitor safety, demonstrate real-world comparative effectiveness, advance discovery, and more. Contact us for more information.
For more details on Truveta Studio, read our approach to analytics whitepaper.
