by Truveta staff | Apr 4, 2024 | Data
US spending on medical devices and in-vitro diagnostics totals more than $199 billion a year, with most of the costs associated with clinical development. Label expansion provides a pathway for recouping costs associated with the device development process by...

by Truveta staff | Apr 2, 2024 | Data
A common misconception is that drugs and devices must gain explicit approval from the FDA for each specific use before healthcare providers can employ them. However, a practice known as off-label use challenges this notion, revealing a broader landscape of treatment...

by Truveta staff | Jan 31, 2024 | Data
Clinical trials and registries have traditionally been the gold standard for high-quality healthcare data, offering complete longitudinal records across data domains in a clean, analysis-ready format. However, achieving this level of quality involves significant...

by Truveta staff | Jan 23, 2024 | Research
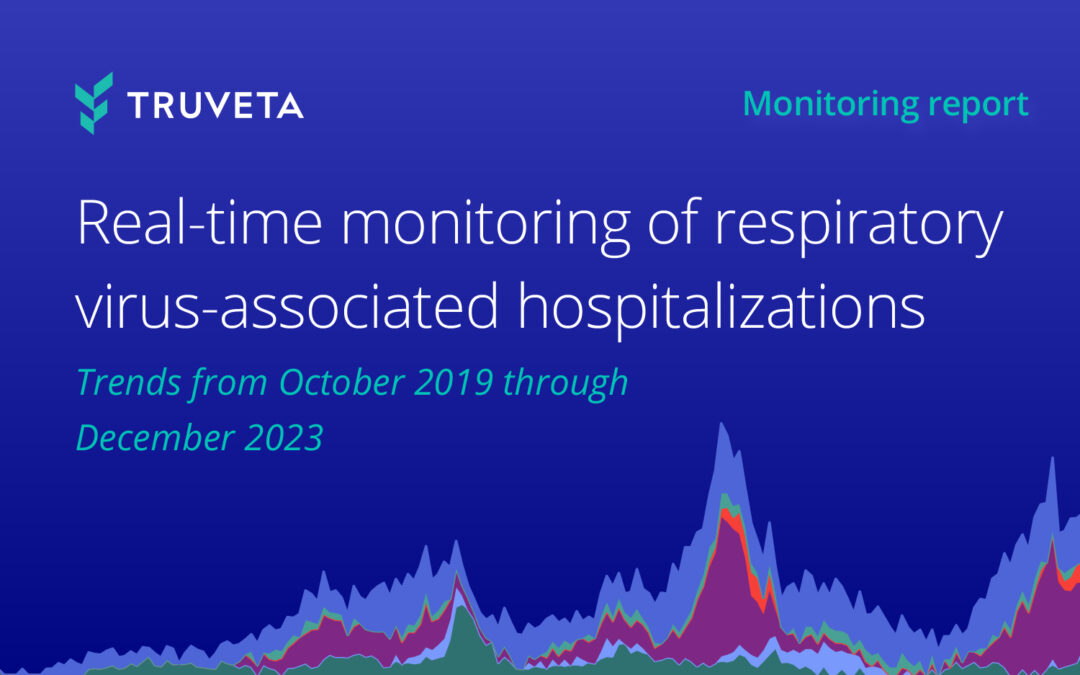
Overall, the rate of hospitalizations associated with respiratory viruses continues to increase (92.8% increase from November 2023 to the end of December 2023). The largest increases in hospitalizations over the past month were associated with influenza (251.7%...

by Truveta staff | Jan 22, 2024 | News
Truveta, a growing collective of 30 health systems with a shared mission of Saving Lives with Data, was awarded a contract with the U.S. Centers for Disease Control and Prevention (CDC) for privacy-preserving record linkage of patient-level real-world healthcare data....