Heart disease is a massive crisis in this country. Ranking as the leading cause of death in the US for more than a century, heart disease takes a life approximately every 33 seconds. That’s about the same time it takes an average person to blink eight times. Although death rates for cardiovascular disease had been steadily falling since 1950 (thanks to reductions in smoking and better detection and treatment options), mortality is now trending upward due to long term increases in obesity and high cholesterol, and more recently due to the COVID-19 pandemic.
For researchers working to better understand the causes, prevention, and treatment for heart disease, the urgency is only increasing. The latest projections, published in the Journal of the American College of Cardiology, expect a “steep rise in cardiovascular diseases by 2060.” Fortunately, improved access to EHR data and the power of advanced AI technology are unveiling previously inaccessible insights to propel discovery and innovation.
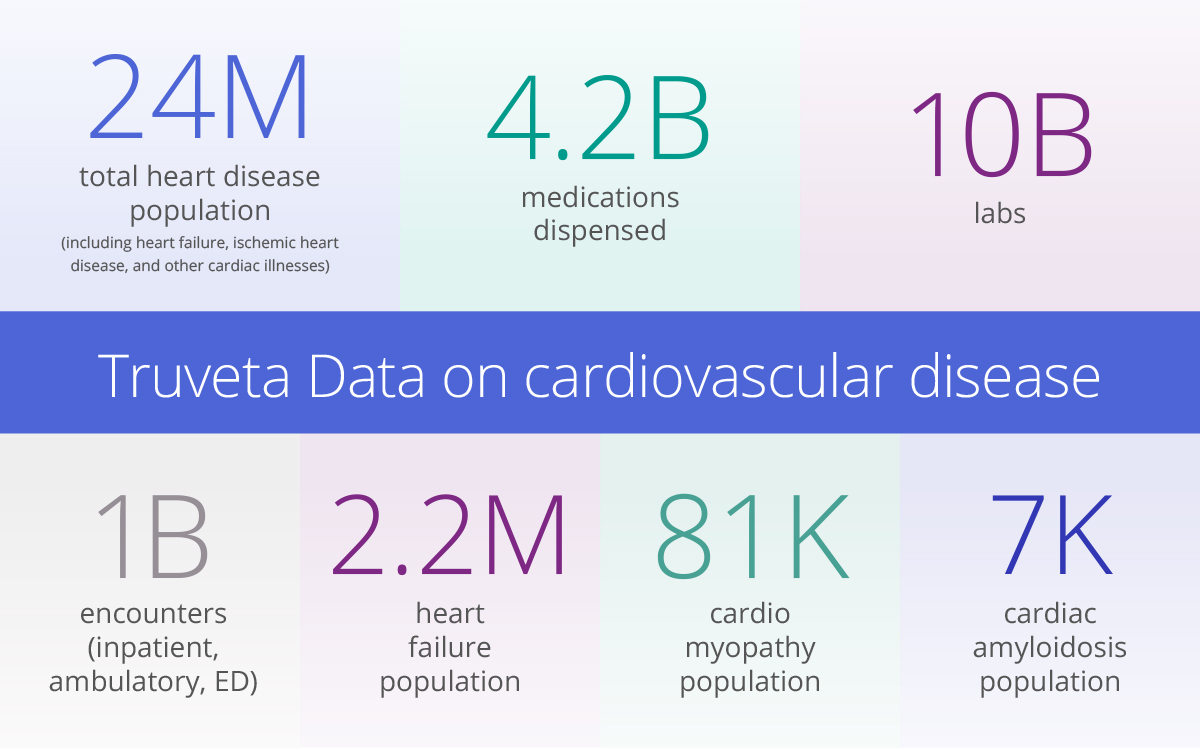
Truveta is the market leader in EHR data for cardiovascular research
Truveta delivers the most complete, timely, and clean EHR data from more than 100 million patients across a growing collective of US health systems. This nationally representative, regulatory-grade data includes full patient medical records, notes, and images, and is linked with claims, SDOH, and mortality data.
Further differentiating this EHR dataset is Truveta’s clinical expert-led AI. The Truveta Language Model is responsible for extracting and structuring previously hidden information from clinician notes and echocardiograms, leading to the availability of more than 56.6 million clinical observations from more than 3.4 million echocardiograms. Never before have these test results been available in a discrete and normalized format for research at this scale.
Read on to discover ways to use AI-extracted echo data, examples of clinical depth within patient-level and device-level data, and more highlights of Truveta’s daily updated cardiovascular disease data.
Using echo data across the product lifecycle
Truveta Language Model extracts and normalizes clinical measures from clinician notes and echocardiograms at scale. By leveraging TLM, we extract precise metrics such as ejection fraction percentages. Access to this information provides researchers with the ability for more advanced and nuanced queries, beyond binary comparisons like “reduced EF” vs. “preserved EF.” These data that have been extracted from echo reports are added to Truveta Data, providing complete clinical context to uncover novel insights.
Echo observations can be used to propel research across the product lifecycle, from tailoring treatment interventions, to assessing real-world effectiveness, to long-term safety monitoring. Having access to echo observations can also help to ensure higher quality comparative effectiveness research. For example, Truveta recently examined the impact of two popular SGLTis (dapagliflozin vs. empagliflozin) on mortality rates and was able to identify baseline LVEF (left ventricle ejection fraction) as a potentially biasing covariate.
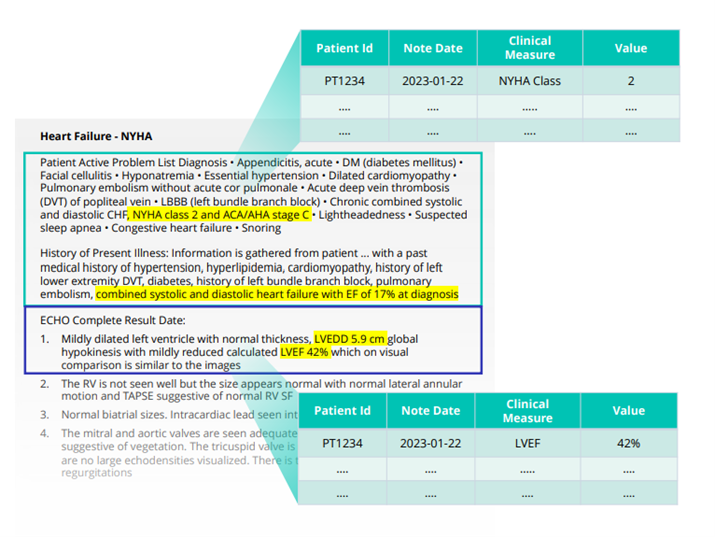
This is just one example of how researchers can leverage this type of AI-extracted detail to ultimately improve patient care and outcomes. Truveta Data now includes clinical observations from cardiac catheterization reports as well, which provide even more data to study conditions like ischemic heart disease and pulmonary hypertension.
Unprecedented patient-level and device-level data
Direct from 30+ health system members and held to stringent data quality standards, Truveta Data also includes lab tests and results, biometric data, genomic biomarkers, UDI level device data, immunizations, biopsy reports, medication dosage, pharmacy fill data, and more – such as results from the KCCQ to analyze patient-reported outcomes at scale. Researchers can also find transplant data, often only available via registries. These registries are often only updated quarterly, whereas Truveta Data is updated every day.
The clinical depth in Truveta Data supports populations with highly specific inclusion/exclusion criteria for any disease, drug, or device. Examples of detailed patient and device-level data available include:
- B- type natriuretic peptide (BNP): common lab/blood test that is marker for fluid status and overload: 5M events
- Body weight, closely tracked in heart failure patients: 22M events
- Left ventricular assist device (LVAD): 694K events
Studying rare cardiovascular conditions using EHR data
Cardiovascular disease as a category is unfortunately common, but there are also many rare conditions that affect the heart. Individual health systems may only have a handful of patients with a particular rare condition, an insufficient population to make meaningful strides in research. Truveta’s breadth and depth of EHR data make it possible to learn from more statistically representative populations.
One such condition is cardiac amyloidosis, a rare disorder where faulty proteins build up in the heart. While treatable, this condition is often underdiagnosed. Amyloidosis is among the concepts Truveta is already extracting from clinician notes to improve diagnostic ability and possibly discover novel treatments. This rare condition has a patient cohort within Truveta Data of 7K. Tafamidis, a medication used to treat amyloidosis, has over 30K events.
Role of SDOH data in understanding heart disease in diverse populations
Heart disease is the number one cause of death for men, women, and all ethnic groups in the US, with non-Hispanic Black Americans suffering from the highest rates of any group. Beyond demographic factors, social drivers of health (SDOH) play a significant role in the disease’s development and outcomes. Truveta Data includes more than 440 attributes on education, income, housing stability, social support, and more so that every study can be a health equity study. Researchers can find answers to questions such as who is getting transplanted, how marital status impacts medication adherence, or which income groups have better outcomes.
Examples of SDOH cohorts available within the heart disease population include:
- Patients with income less than $50K: 6M
- Patients living alone: 9M
- Patients with no vehicle registered: 2M

Saving Lives with Data
Truveta’s Real-World Data (RWD) is currently being used to study a wide-range of CVD topics including a first-of-its-kind comparative study of medical devices for treatment of pulmonary embolism (from Boston Scientific), elevating standard of care for acute decompensated heart failure (from Reprieve Cardiovascular), and racial and ethnic disparities related to maternal heart health (from Truveta Research). With more than 18% of daily clinical care across all 50 states from more than 800 hospitals and 20,000 clinics, Truveta offers the most complete, representative, and timely real-world data to study all types of cardiovascular disease.
Schedule a demo to learn more about how Truveta’s real world data empowers researchers to generate evidence to drive product innovation and clinical advacements.
