From its rapid global escalation in the 1980s to new gene-editing capabilities signaling hope for a potential cure, the history of HIV has been marked by scientific advancement and persistent challenges that underscore the need for enhanced detection, treatment, and understanding.
Data-driven HIV insights with Truveta’s EHR Data
For researchers studying HIV, Truveta provides the most complete, timely, and clean EHR data. Truveta’s regulatory-grade data includes full patient medical records, notes, and images, linked with claims, SDOH and mortality data for more than 100 million patients across the US. Truveta Data is updated daily from more than 30 member health systems to help accelerate time to market and therapy adoption.
Read on to examine the details and potential use cases of the HIV population available within Truveta. You can also explore this population directly in Truveta Studio.
Patient-level data with unmatched clinical depth
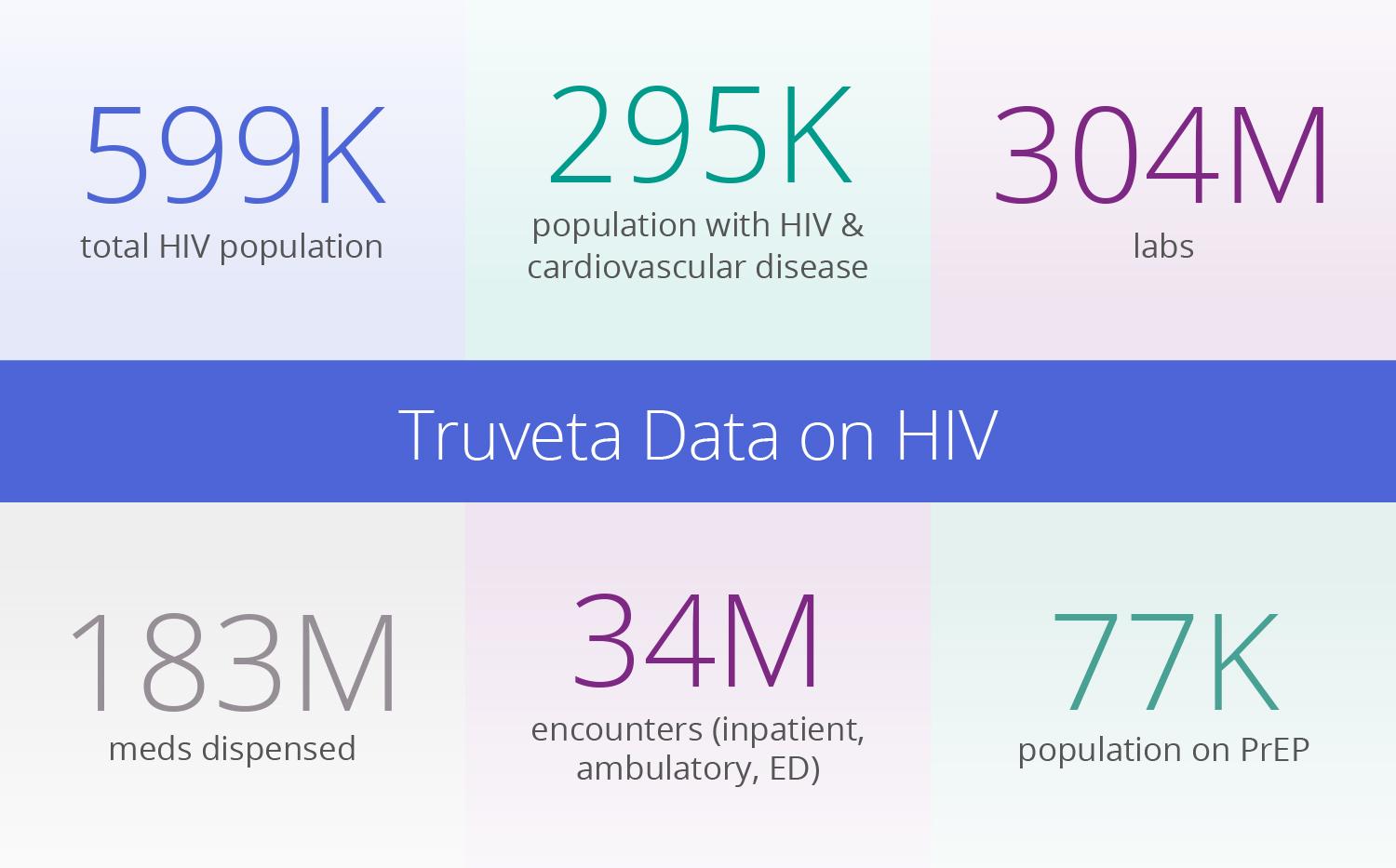
With nearly 600K patient journeys impacted by HIV available in Truveta Data, nearly half of the active US HIV population is available for study. While traditional claims data lacks lab values, symptoms, side effects, and clinical outcomes, Truveta Data provides access to the full medical record and is held to stringent data quality standards.
This clinical depth supports populations with highly specific inclusion/exclusion criteria for any disease, drug, or device. For HIV research, this rich patient-level data includes:
HIV population:
- CD4 count, used to measure immune function: 386K events
- HIV-1 RNA, measure of viral load: 651K events
- Antiretroviral therapy (ART): 7.9M dispenses
- Lipid panel testing (Cholesterol/LDL): 1.8M events
- Liver function testing (AST/ALT): 4M events
PrEP population:
- Cabotegravir, first long-acting injectable HIV treatment: 19K dispense events
- Oral PrEP (taken daily) dispense counts: 2.3M events
- Kidney function (eGFR): 1.2M events
It is well documented that HIV has a disproportionate impact on certain subpopulations, communities, and those with specific risk behaviors. Researchers can also explore more than 440 SDOH attributes, such as the 189K patient journeys within the Truveta HIV population with annual income of less than $50K.
Research possibilities across the HIV patient journey
Further differentiating Truveta Data is the ability to uncover previously hidden insights from more than 5 billion clinical notes. For HIV patients, where strict adherence to a medication regimen is of utmost importance, researchers can discover details around side effects (such as, “daily nausea but tolerating”) in notes along with reasons for switching medications.
With unprecedented access to these clinical notes, integration of SDOH factors, and medication requests, administration, and dispenses, researchers can use Truveta Data to explore:
- Disparities that may exist around newer therapies that may not be covered by insurance
- How patients are adhering to their treatment regimens and what differences are there amongst different groups (racial, ethnic, geographic, income, education) in terms of adherence
- How prescription patterns diverge across demographics and regions, providing a foundation for targeted interventions
- Diagnosis rates and adoption of fourth-generation HIV tests
Example from Truveta Research: Real world prescribing of cabotegravir
For those living with HIV, ART can achieve an undetectable viral load, meaning the virus is not detectable by standard blood tests and cannot be transmitted to sexual partners. Prior to 2021, daily oral pills were the only ART treatments available, but in January 2021, the FDA approved the first extended-release injectable treatment (cabotegravir and rilpivirine).
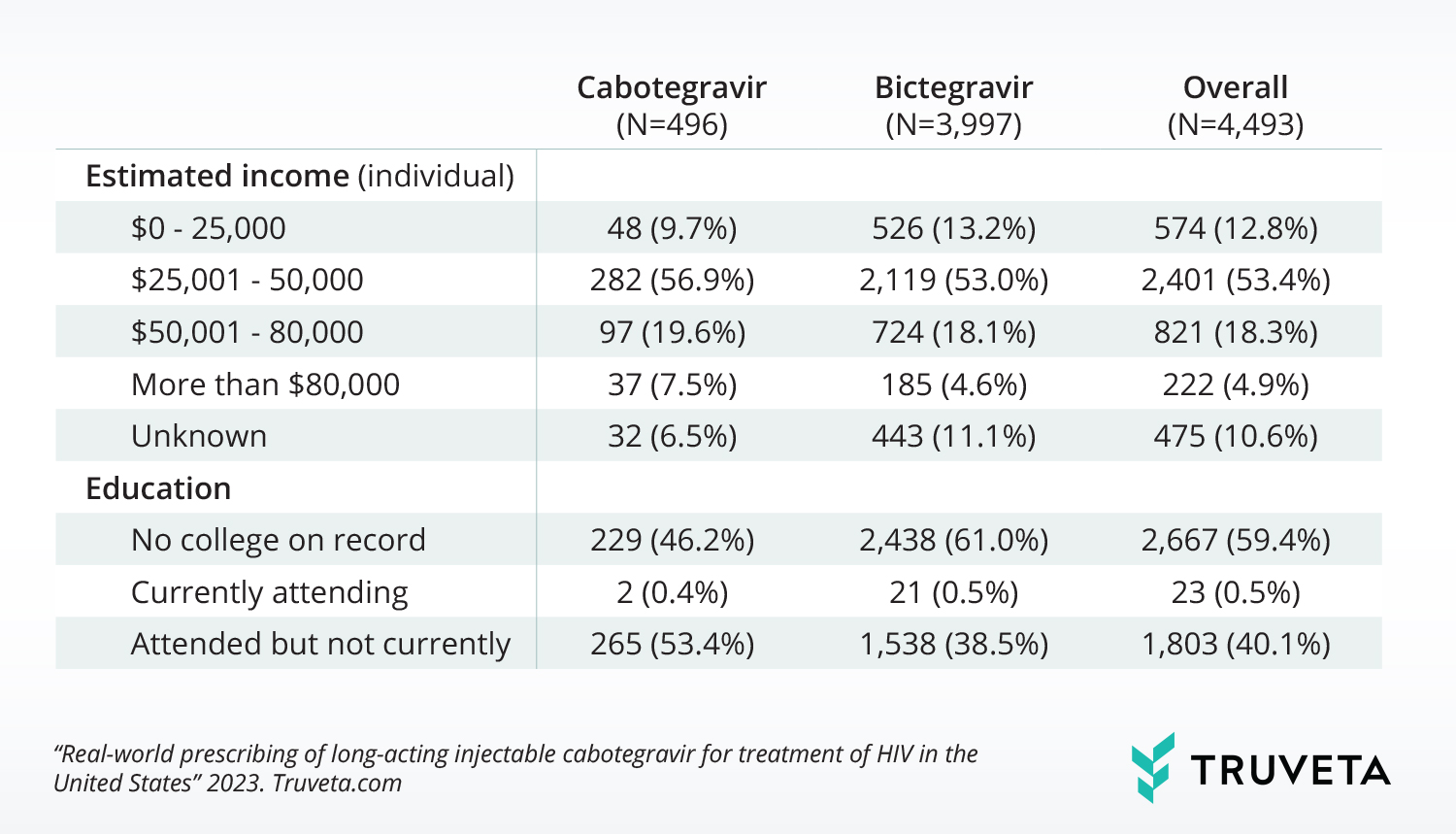
With little known about who is being prescribed the new injectable medication or if there are barriers to access, Truveta Research compared a population of people with first-time cabotegravir and bictagravir (common oral therapy) prescriptions. Using a subset of Truveta Data, they identified patients with HIV with a first-time prescription for either medication between January 2021 and June 2023, and analyzed patient demographics, comorbidities, SDOH, and most recent CD4 count.
Findings showed those with first-time cabotegravir prescriptions are more likely to be younger, have a longer HIV duration, have a higher baseline CD4, have higher individual income, and are more likely to have attended college than those first prescribed bictegravir. Bictegravir is still prescribed over 2x more often, suggesting there may be barriers to accessing cabotegravir for a population with HIV.
This is just one example of how researchers can leverage the clinical depth of Truveta’s patient-level EHR data to improve patient care and outcomes.
Ending the HIV epidemic
As public health officials and scientists race to end the HIV epidemic in this country, new findings have the potential to impact millions of lives worldwide. By leveraging the power of Truveta Data, researchers can speed time to previously inaccessible insights and contribute to a growing understanding of this complex disease.
Interested in learning more about our EHR data and analytics solution?
Explore the Truveta HIV population in detail directly in Truveta Studio.
Request a demo with our team: Contact Us| Truveta
