It’s no secret — yet surprising and tragic — that the United States is one of the most dangerous high-income countries in which to give birth. It’s also unfortunately well known that nearly every maternal health outcome in the United States negatively affects women of color more than white women. One of those conditions impacting new mothers is heart failure. In fact, heart failure in women following delivery is one of the leading causes of death of pregnant women in the United States. One potentially deadly connection is preeclampsia, which is high blood pressure during pregnancy and can be serious or even life-threatening to mother and baby.
Recently Truveta Research explored preeclampsia-related heart failure, seeking to understand how often women with preeclampsia also experience heart failure, and for those who do, the role race and ethnicity might play. In an analysis like this, speed to insight is an imperative. Pregnancy itself creates timing challenges when it comes to research: How many more people will become pregnant, experience pregnancy-related conditions, and give birth, in the nine months it could take a researcher to even begin to create a dataset to begin their research? Then when you layer on the life-threatening nature of preeclampsia and heart failure, there is no time to waste. There must be a faster way.
Speeding time to insight with the most timely, complete, and highest quality data
Traditionally, research studies take several months or years to complete. Researchers must define the research question, establish the cohort criteria, source and normalize the data, and then finally analyze the population to determine the findings. Researchers often face frustrating months-long delays along each step of the process.
Truveta Studio enables a different approach. With the most timely, complete, and highest quality data on US health, Truveta Studio is the first solution to make massive streams of daily clinical data useful for analytics, empowering researchers to ask and answer complex medical questions in days, not years.
“There’s no standard timeline,” said Dr. Nick Stucky, VP of Truveta Research. “Previously this could have taken researchers months to just receive authorization and access the data, let alone make that data useful for analysis. This study took just a few weeks, including less than one week to fine-tune the findings.”
The breadth of the data in Truveta Studio is matched by unparalleled depth, including medical records with full diagnoses, vital signs, lab tests, clinical notes, and images. Truveta data is linked across providers and with daily mortality data and comprehensive social drivers of health data from LexisNexis. Insurance claims fill in the patient journey when medical records are unavailable. The result? A complete, de-identified longitudinal journey for each patient. Data is updated daily from care delivered by more than 28 US health system members, enabling researchers to learn in real time from the most current view of US health. In this study, the data was pulled and analyzed on January 25, 2023, enabling the timeliest view of maternal health possible.
Critically important to a health equity study like this one, the data is also representative. With de-identified patients from all 50 states, Truveta data covers the full diversity of the US across age, geography, race, ethnicity, and gender. A truly representative data set is required to identify accurate trends about the potential impact of race and ethnicity on patient care and outcomes.
Making the complex usable and computable
Ensuring a representative, timely, and deep data set is only the first step. Defining that population who meets the criteria for the study can be a complex, arduous task. In this case, preeclampsia, heart failure, and pregnancy are all terms that must be defined clearly and transparently to ensure clarity, accuracy, and trust in the findings. Yet, that’s far more complex than one might expect.
Truveta Library, a part of Truveta Studio, is comprised of data definitions which leverage the FDA’s Established Pharmacologic Classes (EPC) classification system, so researchers can use previously defined, accurate medical concepts, and reproduce and combine those concepts to speed time to research. Data definitions are shared in Truveta Library to ease the creation of populations for study and to accelerate the accumulation of computable medical knowledge. Truveta Library already contains over 1,500 data definitions contributed by experienced clinical informaticists.
Truveta researchers and clinical informaticists began with a typical research framework document, including the definitions they required. Three of the most important data definitions in this study are also the most complex: heart failure, pregnancy, and preeclampsia. These definitions enable the team to transparently define a condition using all applicable diagnoses, labs, procedures, medications, vaccinations, or devices. Some, like the heart failure data definition, began with prior research. Because the data definition already existed, it was easy to reproduce in Studio.
“The newer the field is, there’s less prior research than in a lot of areas,” Dr. Stucky said. “The data definitions allow us to build off previous work, which saves a great deal of time. The size of the dataset and the populations we can define in seconds is remarkable.”
“Pregnancy is hard to capture because it’s a condition marked by timing,” Raj Brar, MD, MHS, FAMIA said, a Truveta clinical informaticist who worked on creating and modifying the data definitions for this study. “With preeclampsia, we can look at lab results, and build definitions based on diagnoses to capture the specifics on timing.”
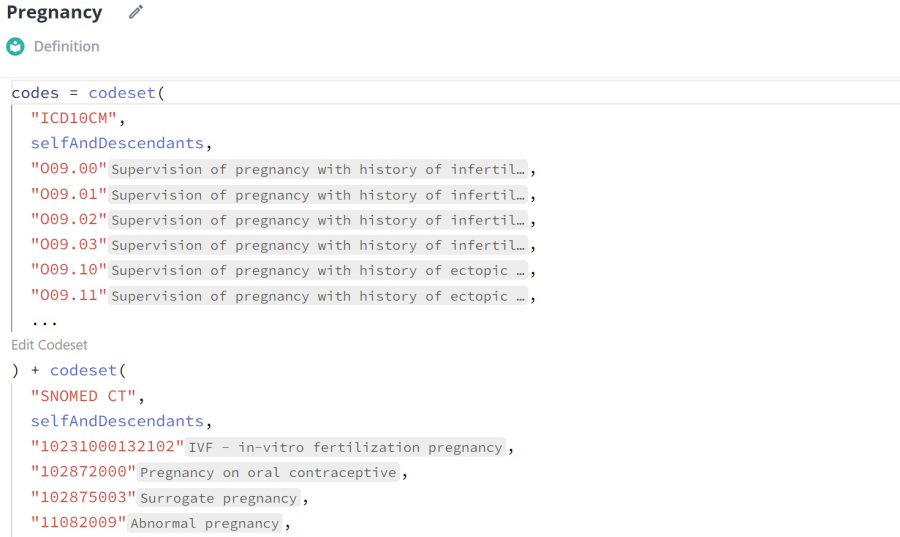
A section the pregnancy data definition in Truveta Studio, with ICD10 and SNOMED codes.
Once the data definition is defined, research can immediately visualize the underlying population in seconds and generate a snapshot to dive deeper into the data for their analysis.
“In the past, researchers would have to identify the normalized codes for their local electronic health record, and health systems don’t typically have this comprehensiveness,” Dr. Brar said. “They’d have more ad hoc, hands-on analysts figuring out which codes were in this database or that database, and then they’d involve clinicians to help them. They can do this research, but even then, they can’t come up with an estimate.”
Like Google searches the Internet, researchers can search data in Truveta for any pre-defined population within a few seconds.
Saving lives with data
Because Truveta Research worked so quickly, they now have timely actionable insights to help inform the care of pregnant patients with preeclampsia. As their results showed, preeclampsia-related heart failure disproportionately impacts Black mothers. These results were highlighted in a New York Times article about new guidelines for treating pregnant women with hypertensive disorders like preeclampsia.
“With Truveta Studio, we were able to reproduce research quickly and identify new trends and disparities to contribute critical new knowledge to this space and drive awareness that can hopefully improve maternal care today, not years from now,” said Dr. Stucky.
Read the preliminary findings and stay tuned for more information about our research, our team, and our tools. For a demo of Truveta Studio, contact us below.
